Abstract
Tuberculosis is an air-borne disease, mostly affecting young adults in their productive years. Here, Ligand-based drug design approach yielded a series of 23 novel 6-(4-nitrophenoxy)-1H-imidazo[4,5-b]pyridine derivatives. The required building block of imidazopyridine was synthesized from commercially available 5,5-diaminopyridine-3-ol followed by four step sequence. Derivatives were prepared using various substituted aromatic aldehydes. All the synthesized analogues were characterized using NMR, Mass analysis and also screened for in vitro antitubercular activity against Mycobacterium tuberculosis (H37Rv). Four compounds, 5c (MIC-0.6 μmol/L); 5g (MIC-0.5 μmol/L); 5i (MIC-0.8 μmol/L); and 5u (MIC-0.7 μmol/L) were identified as potent analogues. Drug receptor interactions were studied with the help of ligand docking using maestro molecular modeling interphase, Schrodinger. Here, computational studies showed promising interaction with other residues with good score, which is novel finding than previously reported. So, these compounds may exhibit in vivo DprE1 inhibitory activity.

Similar content being viewed by others
Introduction
Tuberculosis is major threat for mankind from past several decades. Tuberculosis is the leading cause of death from infectious diseases [1]. Although the number of tuberculosis cases decreased during the twentieth century, the emergence of HIV and the incidence of multiple-drug resistance (MDR) have increased the difficulty of treating many new cases. Despite of the efforts taken to improve the outcome of tuberculosis care, the discovery of new antibiotics against the causative agent is not in a race of expected progress [2, 3]. With this, new and more effective molecules with novel mechanism of action are required to discover which may shorten the treatment, improve patient adherence, and reduce the appearance of resistance [4].
Furthermore, Mycobacterium tuberculosis (M. tuberculosis) has also proven one of the world’s most dreadful human pathogen because of its ability to persist inside humans for longer time period in a clinically inactive state. Roughly 95% of the general population who infected (33% of the worldwide population) built up an inert infection [5, 6]. The current available vaccine, Mycobacterium bovis Bacillus Calmette–Guerin (BCG). M. tuberculosis stimulates a solid response, however it has ability to oppose the body’s activities to kill it and regardless of the possibility of underlying disease is effectively controlled. The discovery of drugs with novel mechanism of action is required because of the expanding number of MDR, which are strains of M. tuberculosis that are resistant to both isoniazid and rifampicin (first line therapy), with or without protection from different medications, broadly extensively drug resistance (XDR) and MDR strains additionally resistant to any fluoroquinolone and any of the second-line against TB injectable medications (amikacin, kanamycin, or capreomycin). Imidazopyridine derivatives are very important, versatile motifs with significant applications in medicinal chemistry [7,8,9].
The imidazopyridine scaffold was found in a number of marketed drug formulations and drug candidates such as antiulcer-zolimidine [10] and tenatoprazole [11,12,13], sedative-zolpidem [14], anxiolytic-saripidem [15] and necopidem [16, 17], analgesic and antipyretic-microprofen [18], cardiotonic-olprinone [19, 20], anti-tumour-3-deazaneplanocin A [21, 22]. Fortunately, 3-deazaneplanocin A was also found to be effective for the treatment against Ebola virus disease [23,24,25,26]. In addition, compounds containing the moiety imidazopyridine have significant biological applications such as antimycobacterial, anticoccidial, antimicrobial [27,28,29,30,31,32,33,34].
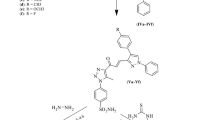
In other words, the therapeutic application of imidazopyridine is not restricted, and need to explore to the fullest for the betterment of mankind. Here, we are looking forward to uncover the potential of 1H-imidazo[4,5-b]pyridine nucleus as a biological agent, hence, we thought to synthesize 6-(4-nitrophenoxy)-2-substituted-1H-imidazo[4,5-b]pyridine derivatives. Purposely 4-nitrophenoxy substitution was chosen on 6th position of 1H-imidazo[4,5-b]pyridine ring because it was proved that the nitro containing compounds shown binding with cys387 residue of DprE1 enzyme protein.
Reports of World Health Organisation (WHO) in past couple of years pointed out that, the global burden of tuberculosis is increasing drastically across the globe. With this threatening scenario of tuberculosis infection, it’s a strict need to search promising drugs which will effectively kill the mycobacterium within short duration of time. Here, we have made an attempt to synthesized novel compounds of imidazopyridine series for antitubercular activity, which may target particularly decaprenyl-phosphoryl-ribose 2′-epimerase (DprE1) enzyme (DprE1 is a novel target for which no drug is available in market till date) in search of novel lead for antitubercular drug discovery to serve the society.
Experimental
Chemistry
All the chemicals were obtained from Sigma Aldrich, Germany, Merk India, Rankem India, Loba Chemi, India, Signichem laboratories, India. Melting points (m.p.) were detected with open capillaries using Veego Melting point apparatus, Mumbai India and are uncorrected. IR spectra were recorded on IR Affinity-1S (FTIR, Schimadzu, Japan) spectrophotometer. 1H and 13C NMR was obtained using a JEOL, JAPAN ECZR Series 600 MHz NMR Spectrometer using tetramethylsilane (TMS) as internal standard. All chemical shift values were recorded as δ (ppm), coupling constant value J was measured in hertz, the peaks are presented as s (singlet), d (doublet), t (triplet), dd (double doublet), m (multiplet). The purity of compounds was controlled by thin layer chromatography (Qualigens Fine Chemicals Mumbai, silica gel, GF-254).
General procedure for synthesis
5,6-Diaminopyridine-3-ol and different substituted aromatic aldehydes were commercially available. The process of four step reaction sequence was initiated with acetylation of 5,6-diaminopyridine-3-ol 1 which on reaction with acetic anhydride forms compound 2 by nucleophilic substitution reaction [35]. To increase the reactivity of –OH, the hydroxyl group, it is converted to its potassium salt by stirring compound 2 [36] with K2CO3 in dimethylformamide (DMF) for 3–4 h and then, p-chloronitrobenzene diluted in DMF (1:1) was added drop-wise for 1 h [37]. Again reaction mixture was stirred for 2–3 h to obtained compound 3. Further, the reactions mixture was poured in cold 10% sodium hydroxide [38, 39]. The compound 4 was precipitated out which further recrystallized by ethanol [40,
Antitubercular activity
In vitro anti-tubercular studies for determination of minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) The in vitro studies were carried out on M. tuberculosis H37Rv (ATCC 27294) strain to determine MIC of test compounds with Isoniazid as standard reference. Microbial culture was developed on Middlebrook 7H9 broth supplemented with 0.2% glycerol, 0.05% Tween 80 (Sigma), and 10% albumin dextrose catalase. The test compounds were prepared as stock and dilutions in DMSO and MIC was determined by microdilution technique. After the incubation period of culture in presence or absence of test compounds, the viability of bacteria was determined by observing the colour change from blue to pink of resazurin mixture which acts as indicator of the inhibitory activity and potency. It was found that compounds 5c, 5g, 5i and 5u exhibited MIC between 0.5 and 0.8 µM which is found very close to the standard reference Isoniazid with MIC of 0.3 µM. The compounds with good MIC were found to be substituted with nitro, methoxy, hydroxyl and halogens like fluorine, chlorine, bromine. Earlier it was reported that nitro group containing compounds inhibit DprE1 selectively due to conversion of the nitro to reduce form and then its interaction with Cys387 residue. Here, we didn’t observed any interaction of synthesized compounds with Cys387 but most of compounds exhibited good docking score with better In vitro antitubercular activity. Furthermore, we have plan to test the compounds with subject to enzyme specific DprE1 inhibitory activity.
Conclusion
We have reported a series of 6-(4-nitrophenoxy)-1H-imidazo[4,5-b]pyridine Derivatives 5a–w. Newly synthesized compounds were tested for their In vitro antitubercular activity on the virulent strain H37RV of M. tuberculosis. Few compounds have shown attractive antitubercular activity, among the active compounds, 5c, 5g, 5i and 5v have shown good potency towards M. tuberculosis strain. Molecular docking studies were also carried out using the reported crystal structure of DprE1, we studied flexible binding modes for the synthesized compounds in comparison with the cocrystal reference molecules TCA1 and BTZ043. Interestingly, same compounds (5c, 5g, 5i and 5v) were come up with excellent docking score. Knowledge from the molecular docking studies emphasize that further modifications are also possible in the series of molecules to develop better compounds for potential DprE1 inhibitory activity. Previously, it was reported that nitro group gets reduced and forms adduct with Cys387 to exhibit DprE1 inhibitory activity. Current molecular docking studies strikes on interactions of synthesized chemical structures with various amino acid residues but does not showed any interaction with Cys387 residue but shown excellent docking score. These compounds may exhibit DprE1 inhibitory activity. This information on ligand binding in active site from crystal structure can be utilised for further medicinal chemistry efforts to study enzyme specific inhibition study (Additional file 1).