Abstract
Background
There are high rates of sexually transmitted infections (STIs) in ethnically diverse, sexually active students aged 16–24 years attending London further education (FE) colleges. However, uptake of chlamydia screening remains low. The TnT study aims to assess the feasibility of conducting a future trial in FE colleges to investigate if frequent, rapid, on-site testing and treatment (TnT) reduces chlamydia rates. This article presents the statistical analysis plan for the main study publication as approved and signed off by the Trial Management Group prior to the first data extraction for the final report.
Methods/design
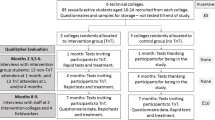
TnT is a cluster-randomised feasibility trial conducted over 7 months with parallel qualitative and economic assessments. Colleges will be randomly allocated into the intervention (TnT) or the control group (no TnT). Six FE colleges in London will be included. At each college for 2 days, 80 consecutive sexually active students aged 16–24 years (total 480 students across all six colleges) will be recruited from public areas and asked to provide baseline samples. One and 4 months after recruitment intervention colleges will be visited on two consecutive days by the TnT team where participating students will be texted and invited to come for same-day, on-site, rapid chlamydia testing and, if positive, treatment. Participants in the control colleges will receive ‘thank you’ texts 1 and 4 months after recruitment. Seven months after recruitment, participants from both groups will be invited to complete questionnaires and provide samples for TnT. All samples will be tested, and same-day treatment offered to participants with positive results. Key feasibility outcomes include: recruitment rates, testing and treatment uptake rates (at 1 and 4 months) and follow-up rates (at 7 months).
Trial registration
ISRCTN 58038795. Registered on 31 August 2016.
Similar content being viewed by others
Background
There are high rates of sexually transmitted infections (STIs) in ethnically diverse, sexually active students aged 16–24 years attending London further education (FE) colleges [1,2,3,4], with around 8% testing positive for Chlamydia trachomatis. However, uptake of chlamydia screening remains low: below 30% annually in 16–24-year-olds in England [4, 5]. Although chlamydia and gonorrhoea primarily affect young people, the consequences of infection such as infertility, chronic pelvic pain or epididymitis can last a lifetime. It is estimated that 10–16% of women with untreated chlamydia will develop clinical pelvic inflammatory disease of whom 8% will have an ectopic pregnancy and 11% will suffer from tubal-factor infertility [6]. The cost of chlamydia and gonorrhoea to the NHS is estimated to be over £100 million each year.
Barriers to reducing chlamydia rates include low uptake of testing by those most at risk [5, 7] (such as teenagers, people from ethnic minorities and people who are socioeconomically deprived), and long delays in receiving a positive diagnosis or attending for treatment [8, 9]. Introducing rapid, on-the-spot chlamydia tests and treatment into the community could make it easier for young people to get tested and treated faster before they can pass on their infection. It might also prevent complications [10, 11]. These novel tests can have 99% sensitivity and 99.4% specificity [12], and studies have demonstrated their feasibility in remote communities [13]. However, there have been no UK trials of rapid sexually transmitted infection (STI) tests and same-day, on-site treatment in non-healthcare settings.
Objective
To assess the feasibility of conducting a future trial in further education (FE) colleges to investigate if frequent, rapid, on-site testing and treatment (TnT) reduces chlamydia rates in sexually active male and female students aged 16–24 years.
Methods and design
TnT is a cluster-randomised feasibility trial conducted over 7 months with parallel qualitative and economic assessments. The outcome is measured within one academic year to optimise follow-up.
Both the current feasibility study and a future, definitive trial will be cluster randomised. This is to avoid contamination, which may arise if students were individually randomised within the same college, as sexual partners could potentially be allocated to different groups. The cluster design also reflects how TnT would be rolled out in practice, with college visits once each term.
Six FE colleges in London will be included. Colleges were included based on their high proportion of black and ethnic minority students and an even gender split, as well as their close proximity to St George’s, University of London (SGUL) [3]. At each college, for 2 days, 80 consecutive sexually active students aged 16–24 years will be recruited from public areas (total 480 students across all six colleges). Research assistants will approach students in common room areas. The students will be asked if they are willing to help with research on sexual health. Potentially eligible students will be invited to come to the study table where recruiters will explain that as the study is about chlamydia and sexually transmitted infections, only students who have had penetrative sexual intercourse should consider taking part. Those who are interested will be given a patient information sheet and consent form to read and encouraged to ask questions.
Exclusion criteria
-
Students who self-report never having had penetrative sexual intercourse
-
Students with severe learning disability as identified by college identification badges
Randomisation will take place once recruitment is completed and baseline data collected for all colleges. Colleges will be randomly allocated into the intervention (TnT) or the control group (no TnT) in a 1:1 (i.e. equal allocation) ratio by the trial statistician. The randomisation will be constrained to ensure that three colleges are allocated to each group. Recruitment of colleges and participants will take place prior to group allocation to ensure allocation concealment and prevent selection bias, and, therefore, the baseline data collection from students will be blind to treatment group.
Participants will be asked to provide samples (urine for males and self-taken vaginal swabs for females) at baseline and after 7 months and to complete questionnaires on sexual lifestyle and healthcare use at both time points. Questionnaire data will be collected at the college using encrypted tablet computers. As a contingency, paper questionnaires will be used as back up. All participants will be informed that baseline samples will not be tested for 7 months and will be advised to get screened separately from the study in the event that they are allocated to the control group.
One month after recruitment, each intervention campus will be visited on two consecutive days by the TnT team. (These will be the same days of the week as at recruitment to optimise participating student attendance.) The 80 participating students in each campus will be invited to provide a sample, but this time the sample will be tested immediately on site using the Cepheid GeneXpert system which takes 90 min. Participants will be given a card designed by the user group containing information about STIs, and links to the Brook sexual health website: www.brook.org.uk. Negative results will be sent to participants by text. Participants with positive chlamydia results will be telephoned and invited to come to the college nurse’s room for treatment, partner notification, advice and follow-up by a visiting dispensing (under Patient Group Directive) nurse health adviser. Participants with infections will be asked to bring any sexual partners who attend the college so they can also be tested and treated. The nurse health adviser will arrange for participants who are positive for gonorrhoea to be reviewed by a clinician on the same or the next day at a genitourinary medicine (GUM) clinic. This is for clinical examination, so that repeat samples can be taken to evaluate gonorrhoea resistance to antibiotics, for intramuscular injection of antibiotics and for partner notification. All participants in the three intervention campuses will be invited to provide repeat samples for on-site TnT 4 months after recruitment (i.e. the next college term), when all the procedures described above will be repeated.
Participants from the three control colleges will not get TnT but will receive texts 1 and 4 months after recruitment thanking them for being in the study.
All participants will be asked to provide samples and to complete questionnaires at college at 7 months. Testing at 7 months is required in the proposed full trial to calculate the main outcome (prevalence of chlamydia), and is included here to test the feasibility of collecting these data. Treatment will also be offered to those diagnosed with infection at 7 months. This is not part of the assessed intervention, but is offered to enhance screening uptake, and participation in general among the control group. In addition, at the end of the study, stored baseline samples will be tested using standard tests, and participants with positive results will be contacted by the health adviser. Those not attending for follow-up will be sent an SMS text with a link to the final questionnaire to be completed online. They will also be telephoned and offered the opportunity to provide a sample for routine (not rapid) testing either in college at a prearranged time or by post.
Outcomes
-
1.
The outcomes of this study include key values to inform feasibility, sample size and timescales of a full trial of TnT in FE colleges. These include:
-
(a).
Recruitment rates and associated outcomes:
-
Of the total number of students assessed for eligibility the proportion who are eligible are asked to participate in the study
-
Of those eligible the proportion recruited to the study
-
The time to recruit 80 students at each college
-
Age, gender and ethnicity of students recruited versus students not recruited
-
-
(b).
Testing and Treatment uptake rates (1 and 4 months after recruitment) (intervention colleges only):
-
Of the total number of students recruited in the intervention group the proportion that return at 1 month (and 4 months) and provide a sample for testing
-
Of those tested, the proportion with positive test results and the proportion treated
-
The time from test to informing the participating student of the result
-
The time from test to treatment of positives
-
The number of partners confirmed treated per index case
-
-
(c).
Follow-up rates (at 7 months):
-
Of the total number of students recruited the proportion that return at month 7 and provide a sample for testing
-
Of those tested, the proportion with positive test results and the proportion treated
-
Of the total number of students recruited, the proportion that complete the final questionnaires (including data on healthcare usage)
-
-
(d).
Prevalence of chlamydia in participants at each college at baseline and at 7 months
-
(a).
-
2.
A perspective on the acceptability of TnT in FE colleges emerging from qualitative interviews, including barriers and facilitators to uptake and possible harms. This will be described in more detail elsewhere.
-
3.
Estimate of the cost per person screened and treated in TnT versus usual care. Detailed analysis plans for these health economic assessments will be documented separately by the trial’s health economist.
Sample size calculation
Assuming a 30% recruitment rate [14], 1600 students will be approached to recruit 480 overall (80 per college across six colleges: three intervention colleges and three control colleges). Estimates of testing uptake at 1 and 4 months (intervention colleges only) will be based on 240 students, and at 7 months will be based on 480 students (all colleges).
Teare et al. recommend that 60 to 100 subjects are sufficient to estimate an event rate with acceptable precision in a feasibility study [15]. Prevalence of chlamydia at baseline will be estimated separately for each of the six colleges (80 students per college), and these prevalence figures will be used to inform the intraclass correlation coefficient (ICC), required for the sample size calculation for the main study. From our previous research involving 11 colleges, the ICC was estimated to be 0.005 (95% confidence interval − 0.013 to 0.026) [3]. Adding data from another six colleges will improve the precision of this ICC, reducing the width of the confidence interval by around 20%.
Assuming 70% followed up at 7 months, final estimates of chlamydia prevalence would be based on 168 students in each of the intervention and control groups [3]. The study is not powered to find a statistically significant difference in chlamydia rates between groups, but may provide useful information on possible effect size to inform future sample size calculations.
Statistical analyses
Trial profile
The flow of participants will be displayed in the Consolidated Standards of Reporting Trials (CONSORT) flowchart as shown in Fig. 1 [16].
Data management and quality assurance
Contact details will be collected on paper consent forms which will then be entered and stored on an encrypted Access database by authorised research personnel. Attendance and laboratory data will be initially recorded on paper worksheets and then entered, by designated research personnel, to an online database (REDCap) [17] hosted by SGUL. Questionnaire data will be entered electronically using the REDCap mobile application on encrypted tablets where tablets are available, or on paper as a back-up. At the end of the visit to each college, the data will be uploaded or entered to the SGUL servers by designated research personnel.
Research coordinators and the trial manager will periodically perform basic checks, such as examining for improbable values and data completeness, as well as random checks on the data. Anomalies will be explored and checked with original source data.
After the last patient has been followed up, all queries resolved and all data fields completed with data or missing data codes, the database will be locked for final analysis and this process will be overseen by the trial statisticians.
General analysis principles
The analysis principles outlined will be followed as closely as possible in the analysis and reporting of trial data; the statistical analysis plan is not intended to restrict exploratory or other sensible and standard reporting practices. There are no plans for any formal comparisons between treatment groups until final database lock. Analysis will be undertaken by the trial statistician.
Since this a feasibility study no statistical significance testing will be performed. Any confidence intervals presented will be two-sided at the 95% confidence level.
Baseline characteristics
Baseline descriptions of students recruited and tested will be presented by treatment group: including means and standard deviation or numbers and proportions as appropriate. This will include demographic characteristics, history of sexual behaviours and chlamydia and gonorrhoea status (a full list is available in Appendix). These summaries will be based on observed values and the number of missing observations for each characteristic will be reported.
Main outcomes
Recruitment rates (at baseline)
-
1.
We will assess eligibility rates by calculating the proportion of students who were eligible and were asked to participate in the study out of the total number of students who were assessed for eligibility. Similarly, the proportion of students who were recruited to the study out of those eligible will be calculated. All proportions will be presented with corresponding 95% confidence intervals (CIs).
-
2.
The time taken to recruit 80 participants at each college will be described. We will calculate the mean and standard deviation or medians and inter-quartile ranges as appropriate of the time taken to recruit 80 participants at each college.
-
3.
Age, gender and ethnicity at baseline will be presented separately for those recruited to the study and those eligible but not recruited. We will present means and standard deviation or numbers and proportions as appropriate.
Testing and treatment uptake rates, in intervention colleges only (1 and 4 months after recruitment)
-
1.
The number and proportion of participants in the intervention group who return at 1 month and provide a sample out of the total recruited to the intervention group will be presented. Of those tested, the number and proportion of participants with positive test results and the number and proportion of participants treated will be presented. These analyses will be repeated for the 4-month visit. Corresponding 95% CIs will be presented. Where available, the reasons for non-attendance will be summarised
-
2.
Time from test to treatment of positives will be described. Of the participants who attended the 1-month intervention visit, were tested and received a positive test result we will calculate the mean and standard deviation or medians and inter-quartile ranges as appropriate of the time between the sample being taken and the time treatment was received. This will be repeated for the 4-month visit.
-
3.
The number of partners confirmed treated per index case will be described. In addition, we will report the number of partner notifications raised and addressed, the number of participants who confirm partner treated, the number of participants referred to a GUM clinic and, therefore, partner notification responsibility passed to clinic and the number of participants with no information regarding treatment.
Follow-up rates (at 7 months)
-
1.
The number and proportion of participants who return at month 7 and provide a sample out of the total recruited will be presented by treatment group. Of those tested, the number and proportion of participants with positive test results and the number and proportion of participants treated will be presented. Corresponding 95% CIs will be presented.
-
2.
The number and proportion of participants completing the final questionnaire will be presented. Descriptions of participants’ responses by treatment group will be presented (including data on healthcare usage), by means and standard deviation or numbers and proportions as appropriate. We will present the number of repeat follow-up attempts for non-attendees at 7-month follow-up.
Prevalence of chlamydia in participants at each college at baseline and at 7 months
Of those tested, the number and proportion of participants with positive test results will be presented for each college at baseline and at 7 months.
Sensitivity analysis
Missing data analysis
As this is a feasibility study the levels of data completion and follow-up rates are important feasibility outcomes. Therefore, no formal analysis will be undertaken to account for missing data. All summaries will be based on observations only and the number of missing observations for each characteristic will be reported.
Harm data
Participating student and college staff views of potential harms of on-site rapid tests and treatment will be sought and examined in the qualitative interviews. Analysis details will be described elsewhere.
Trial status
Recruitment was completed in October 2016. Final follow-up concluded in August 2017.
Conclusion
Findings from this study are intended to assess the feasibility of running a future definitive trial to investigate whether a FE college-based TnT model leads to a reduction in prevalence of chlamydia. In this article, we have described the TnT statistical analysis plan which provides details about how data from the TnT feasibility trial will be analysed. The protocol for the trial is published by Kerry-Barnard et al. (in press). By publishing our statistical analysis plan, we believe that we will ensure a more balanced, accurate and complete report of our final results.
Abbreviations
- CI:
-
Confidence interval
- CONSORT:
-
Consolidated Standards of Reporting Trials
- FE:
-
Further education
- GUM:
-
Genitourinary medicine
- ICC:
-
Intraclass correlation coefficient
- REDCap:
-
Research Electronic Data Capture
- SGUL:
-
St George’s, University of London
- STI:
-
Sexually transmitted infections
- TnT:
-
Test n Treat
References
Oakeshott P, Aghaizu A, Reid F, Howell-Jones R, Hay PE, Sadiq ST, et al. Frequency and risk factors for prevalent, incident, and persistent genital carcinogenic human papillomavirus infection in sexually active women: community based cohort study. BMJ. 2012;344:e4168.
Oakeshott P, Aghaizu A, Hay P, Reid F, Kerry S, Atherton H, et al. Is Mycoplasma genitaliium in women the ‘new chlamydia’? Community-based prospective cohort study. Clin Infect Dis. 2010;51:1160–6.
Oakeshott P, Kerry S, Aghaizu A, Atherton H, Hay S, Taylor-Robinson D, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. Br Med J. 2010;340:1642.
Aghaizu A, Reid F, Kerry S, Hay PE, Mallinson H, Jensen JS, et al. Frequency and risk factors for incident and redetected Chlamydia trachomatis infection in sexually active, young, multi-ethnic women: a community based cohort study. Sex Transm Infect. 2014;90:524–8.
National Chlamydia Coalition. Getting more young women screened for chlamydia: findings from qualitative research, vol. 3; 2011. p. 1–17.
Public Health England. Opportunistic chlamydia screening of young adults in England. London: Public Health England; 2014.
van den Broek IV, van Bergen JE, Brouwers EE, Fennema JS, Gotz HM, Hoebe CJ, et al. Effectiveness of yearly, register based screening for chlamydia in the Netherlands: controlled trial with randomised stepped wedge implementation. BMJ. 2012;345:e4316.
McClean H, Sullivan AK, Carne CA, Warwick Z, Menon-Johansson A, Clutterbuck D, on behalf of the National Audit Group of the British Association for Sexual Health and HIV. UK national audit against the key performance indicators in the British Association for Sexual Health and HIV Medical Foundation for AIDS and Sexual Health Sexually Transmitted Infections Management Standards. Int J STD AIDS. 2012;23(10):742–7.
Horner PJ. Azithromycin antimicrobial resistance and genital Chlamydia trachomatis infection: duration of therapy may be the key to improving efficacy. Sex Transm Infect. 2012;88(3):154–6. https://doi.org/10.1136/sextrans-2011-050385.
Adams EJ, Ehrlich A, Turner KM, Shah K, Macleod J, Goldenberg S, et al. Map** patient pathways and estimating resource use for point of care versus standard testing and treatment of chlamydia and gonorrhoea in genitourinary medicine clinics in the UK. BMJ Open. 2014;4(7):e005322.
Turner KM, Round J, Horner P, Macleod J, Goldenberg S, Deol A, et al. An early evaluation of clinical and economic costs and benefits of implementing point of care NAAT tests for Chlamydia trachomatis and Neisseria gonorrhoea in genitourinary medicine clinics in England. Sex Transm Infect. 2014;90(2):104–11.
Gaydos CA, Van Der Pol B, Jett-Goheen M, Barnes M, Quinn N, Clark C, et al. Performance of the Cepheid CT/NG Xpert Rapid PCR Test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2013;51(6):1666–72.
Guy RJ, Natoli L, Ward J, Causer L, Hengel B, Whiley D, et al. A randomised trial of point-of-care tests for chlamydia and gonorrhoea infections in remote Aboriginal communities: Test, Treat ANd GO- the ‘TTANGO’ trial protocol. BMC Infect Dis. 2013;13:485.
Balendra A, Cousins E, Lamplough H, Oakeshott P, Majewska W, Kerry SR. Pilot study for the ‘Test n Treat’ trial of on-site rapid chlamydia/gonorrhoea tests and same day treatment. Sex Transm Infect. 2017;93(4):283.
Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials. 2014;15:264.
Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Ann Intern Med. 2010;152 Epub 24 March
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Acknowledgements
We are very grateful to staff and students at the following London FE colleges: Kingston College, Lambeth College, Lewisham Southwark College (LESOCO) and South Thames College (Merton and Wandsworth campuses).
Funding
The research project is funded by NIHR Research for Patient Benefit: PB-PG-1014-35007. The funding body will have no role in the design of the study, the collection, analysis or interpretation of the data, or the write-up of the manuscript.
Author information
Authors and Affiliations
Contributions
RP and FR are the trial statisticians and drafted and finalised the first version of the statistical analysis plan. FR was the statistician on the grant application. PO is a professor of general practice and is the chief investigator for the study. SKB is the trial manager and a co-investigator on the study. PO, SKB and FR with others designed the study and obtained the funding. All authors commented on initial drafts and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was granted by the NRES Committee Bromley ref. 15/LO/1929. All patients are to provide written informed consent prior to randomisation.
Competing interests
The authors declare that they have no competing interests.
Appendix
Appendix
Baseline characteristics
The following baseline characteristics will be summarised by treatment group:
-
1.
Age
-
2.
Sex
-
3.
Ethnicity
-
4.
Female sexual preference
-
5.
Male sexual preference
-
6.
Age at first sex
-
7.
Number of sexual partners in last year
-
8.
New sexual partner in the past 6 months
-
9.
Type of female contraception
-
10.
Condom use
-
11.
Last sexually transmitted infection (STI) check-up
-
12.
STI history
-
13.
Antibiotics received for an infection in the last 2 weeks
-
14.
Which antibiotics
-
15.
Any symptoms in the past 6 months (female)
-
16.
Any symptoms in the past 6 months (male)
-
17.
Smoker status
-
18.
Vape
-
19.
Alcohol consumption in past month
-
20.
Visited GP in the past 6 months
-
21.
Number of GP visits in the past 6 months
-
22.
Reason for GP visit
-
23.
Visited GUM clinic in the past 6 months
-
24.
Number of GUM clinical visits in the past 6 months
-
25.
Visited walk-in clinic in the past 6 months
-
26.
Number of visits to a walk-in clinical in the past 6 months
-
27.
Visited A&E/hospital in the past 6 months
-
28.
Number of visits to A&E/hospital in the past 6 months
-
29.
Attended healthcare facility for sexual health reasons
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Phillips, R., Oakeshott, P., Kerry-Barnard, S. et al. ‘Test n Treat (TnT)’: a cluster-randomised feasibility trial of frequent, rapid-testing and same-day, on-site treatment to reduce rates of chlamydia in high-risk further education college students: statistical analysis plan. Trials 19, 312 (2018). https://doi.org/10.1186/s13063-018-2675-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-018-2675-7