Abstract
Acute kidney injury is a known clinical risk factor for delirium, an acute cognitive dysfunction that is commonly encountered in the critically ill population. In this comprehensive review of clinical and basic research studies, we detail the epidemiology, clinical implications, pathogenesis, and management strategies of patients with acute kidney injury-associated delirium. Specifically addressed are the pathological roles of endogenous toxin or drug accumulation, acute kidney injury-mediated neuroinflammation, and acute kidney injury-associated volume overload as discrete potential biological mechanisms of the condition. The optimization of clinical contributors and normalization of renal function are reviewed as pragmatic management strategies in addition to potential and emerging therapeutic approaches.
Similar content being viewed by others
Introduction
Delirium occurs in approximately 60% of patients with acute kidney injury (AKI) [1]. Substantial clinical evidence suggests a direct pathological role for AKI in delirium. Studies demonstrate that the risk of delirium in patients with AKI significantly increases with worsening renal function [2, 3]. Although the underlying mechanisms for AKI-associated delirium remain largely unknown, several pathobiological processes have been proposed, including accumulation of neurotoxins or deliriogenic drugs due to impaired renal clearance, upregulation of systemic cytokine-mediated neuroinflammatory processes, and volume overloaded conditions.
The goal of this article is to provide a comprehensive review of the breadth of human and animal investigations that examine the clinical and pathophysiological contributions of AKI to delirium. This topic is of particular relevance as AKI affects up to half of critically ill patients [4] and may confer a tenfold increased risk of delirium [5], a condition known to increase morbidity and mortality, prolong hospital stay, and accelerate long-term cognitive decline [6, 7]. We further identify critical knowledge gaps in understanding of underlying biological mechanisms and clinical contributors to inform design of future studies that address novel preventative and therapeutic discoveries.
Background and epidemiology
AKI-associated delirium: clinical burden, long-term outcomes, and risks factors
AKI is estimated to affect more than 10 million people worldwide annually and confer a 1.7–6.9-fold increased risk of hospital mortality [8, 9]. Over half of all critically ill patients develop AKI within 48 h of admission to the intensive care unit (ICU) [4], with mounting preclinical evidence suggesting that AKI often precipitates or exacerbates secondary injury to other organ systems including the brain, heart, and lungs [10,11,12,13]. The most common clinical manifestation of AKI-associated acute brain injury is delirium, which presents as an acute or fluctuating impairment in attention, executive, function, or short-term memory [3, 14,15,16,17,18]. In the short term, delirium is well known to be strongly associated with increased mortality, prolonged hospitalization, and need for intensive medical interventions [1], while persistent cognitive decline is a feared long-term sequela [19].
The Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) study found that patients with delirium who survived their hospital course developed long-term cognitive impairment including 20% of patients whose cognition was similar to that of Alzheimer’s disease [20]. Longer duration of delirium was also found to be an independent risk factor for worse global cognition [20, 21]. In terms of the public health impact, delirium accounts for significant societal and health care costs, with the national burden of delirium on the health care system costing up to $152 billion each year in the USA [22] as a result of prolonged hospital stay, increased treatment costs, and long-term acute care requirements [6]. It is now understood that delirium independently contributes to long-term cognitive decline [19], rather than merely an unmasking of a vulnerable brain substrate.
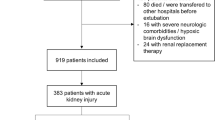
Several studies have identified AKI as a principal risk factor for delirium. In a prospective study of 1487 patients, Zipser et al. found that AKI conferred a tenfold increased risk of delirium (OR 10.01, CI 1.13–88.73, p = 0.039) [5], while the BRAIN-ICU study found that AKI was present in 50% of study days when patients were delirious [23]. Several studies have reported on a direct relationship between AKI severity and delirium risk. In one such study, AKI severity, as measured using the Kidney Disease: Improving Global Outcomes (KDIGO) creatinine criteria, was associated with a significantly increased risk of delirium. Specifically, a 1.5-fold increased risk of delirium was observed with KDIGO stage 2 (OR 1.55; 95% CI, 1.07–2.26) and a 2.5-fold increased risk of delirium with stage 3 (OR 2.56; 95% CI, 1.57–4.16) AKI, while KDIGO stage 1 was not significantly associated with delirium (OR 1.13, 95% CI 0.91–1.41) [2]. Concordant data were reported in a retrospective study that analyzed the medical records of 919 medical ICU patients of whom 41.6% developed AKI and found a higher incidence of delirium with KDIGO stage 2 (66.7%) and stage 3 (66.9%) AKI compared with KDIGO stage 1 (53.6%) [1]. Another prospective cohort study of 304 patients aged 60 or older found elevated creatinine level of > 2 mg/dL (OR 2.1, 95% CI 1.1–4.0) as one of 4 admission risk factors for delirium along with dementia, receipt of benzodiazepines before ICU admissions, and low arterial pH [24].
Further clinical evidence of AKI’s potential role in contributing to delirium was provided by Wan et al. who conducted a single-center case control study in a 30-bed mixed ICU in the UK with 142 cases and 142 matched controls to evaluate AKI-associated hyperactive delirium, a subtype of delirium. In this study, patients with KDIGO stage 3 AKI were five times more likely to develop hyperactive delirium (OR 5.40, 95% CI 2.33–12.51) than those without AKI and that less severe stages of AKI, i.e., KDIGO stage 1 or 2 AKI, were not independently associated with hyperactive delirium [25]. Overall, the dose-dependent relationship between severity of AKI and delirium identified by these studies suggests a direct pathological role, though causality cannot be reasonably established with clinical studies that may be susceptible to the presence of multiple confounding factors including, but not limited to, heterogeneities in use of analgosedation, identification of patients with increased susceptibility to delirium, such as those with preexisting cognitive impairment, and varied environmental triggers of delirium. Furthermore, additional challenges may be posed in differentiating between the more clinically apparent hyperactive from the often-missed hypoactive delirium phenotypes [26], which complicates clinical investigations on risk factors and mechanisms. Future studies should consistently report whether the investigated mechanisms or risk factors relate to hypoactive, hyperactive, or both delirium phenotypes.
A summary of clinical studies that have evaluated AKI as a risk factor in the development of delirium is presented in Table 1.
Pathophysiology of AKI-associated delirium
Several studies have suggested AKI as a key contributor to distal organ dysfunction, not only affecting the brain, but also the heart, lungs, and liver [15, 27]. As with other organs affected by AKI, the pathogenesis of AKI-associated delirium is multifactorial, and hypothesized to be due to direct and indirect pathways, mediated by toxin and drug accumulations [25], electrolyte imbalances [28], impaired volume homeostasis [29], neuroinflammation [13], and imbalances in neurotransmitters [30] (Fig. 1).
Direct neurotoxic effects of AKI from endogenous toxin accumulation
Perhaps intuitively, it is hypothesized that AKI precipitates delirium due to impaired renal clearance of drugs and toxic metabolic waste products. A potential explanation for the direct neurotoxic effect of AKI is from the accumulation of potential uremic neurotoxins. While urea may be considered a surrogate for accumulated neurotoxins, it is not thought to directly contribute to delirium [31]. Although over 130 substances are considered to be potential uremic toxins [32], the uremic guanidino compounds, which include creatinine, guanidine, guanidinosuccinic acid, and methylguanidine, are considered particularly relevant to the pathogenesis of delirium [15, 33, 34]. These compounds are hypothesized to exert their neurotoxic effects through the inhibition of ϒ-aminobutyric acid receptors and activation of N-methyl-d-aspartate (NMDA) receptors which results in neuronal hyperexcitability, abnormal epileptiform activity, and hippocampal injury [33]. While mouse models demonstrate that administration of exogenous creatinine precipitated epileptic activity, these effects were far greater with other guanidino compounds, specifically guanidinosuccinic acid [35].
Given the risk of uremic compounds in inducing neuronal hyperexcitability [33, 35], seizure should be considered in the evaluation of delirium in the setting of AKI. However, it is often clinically challenging, if not impossible, to distinguish between delirium and non-convulsive seizures, due to shared clinical phenotypes and precipitants. Additionally, kidney injury, both acute and chronic, may lead to electrolyte and metabolic disorders like hyponatremia, hypocalcemia, hypomagnesemia, or hypoglycemia that can independently precipitate epileptic activity [36]. Clinically, one case–control study by Oddo et al. found that at least chronic kidney disease was significantly associated with periodic epileptiform discharges. Although AKI did not show statistical significance, there was a trend toward increased risk of periodic epileptiform discharges with AKI (26% with AKI vs 19% without, p = 0.21) [37]. These findings suggest a potential role for electroencephalographic monitoring in AKI-associated delirium to evaluate for seizures as a treatable etiology of delirium-like states, particularly in patients with overt seizure-like semiology, myoclonus, or forced eye gaze deviation.
Direct neurotoxic effects of AKI from drug accumulation
Another common explanation for delirium in the setting of AKI is the accumulation of drugs that are frequently administered in the ICU setting. A common mechanistic theme for the deliriogenic drugs, such as benzodiazepines and certain antibiotics, is their ϒ-aminobutyric acid antagonistic properties [38,39,40,41,42].
Cefepime-induced neurotoxicity is a relatively commonly precipitant of delirium, imposing up to a tenfold greater risk of neurotoxicity when compared to meropenem, and occurring in up to 15% of ICU patients treated with cefepime [42]. The setting of critical illness is believed to create an inflammatory environment that disrupts the integrity of the blood–brain barrier (BBB) [43], thus allowing for the penetration of cefepime into the brain. Given that cefepime is renally cleared, AKI further exacerbates cefepime-induced neurotoxicity due to drug accumulation [42]. If delirium due to cefepime-induced neurotoxicity is suspected, one should investigate for the presence of non-convulsive status epilepticus, which occurs in a quarter of such patients [42]. Adjustment of cefepime dosing or avoidance of cefepime is recommended in AKI to prevent neurotoxicity manifesting as delirium.
Certain classes of drugs, such as opioids and neuropathic agents, confer various degrees of delirium, largely based on their anticholinergic properties. Meperidine, for instance, should be avoided in AKI because its metabolite, normeperidine, may accumulate and result in central nervous system excitation, induce life-threatening seizures, and exacerbate the phenotype of delirium [44,45,46]. An expanded discussion on the risks of delirium from analgosedatives in the setting of AKI will be reviewed below.
AKI-associated systemic and brain inflammation
There is mounting preclinical evidence that AKI induces systemic inflammation, which is considered a key contributing mechanism of delirium [47]. Data from animal models suggest that AKI promotes upregulation of systemic inflammatory processes that contribute to endothelial injury, leukocyte infiltration, release of cytokines and inflammatory mediators, and induction of apoptosis [25, 48, 49]. This pro-inflammatory milieu precipitated by AKI is hypothesized to contribute to multi-organ injury, including the brain [50,51,52]. There is an increased systemic production of interleukin-1 α (IL-1α), IL-1β, IL-6, IL-10, and tumor necrosis factor α (TNF-α), which are implicated in the pathogenesis of delirium [13]. Other animal studies suggest that AKI-induced systemic and neuroinflammation contributes to BBB disruption and altered expression of tight-junctional proteins, resulting in the infiltration of metabolites and toxins into the brain and leading to inflammatory and pathological changes in the brain [34, 53]. This is evidenced by mouse models of AKI that demonstrated extravasation of Evans blue dye into the brain, indicating breakdown of the BBB [13]. In the clinical setting, this process of increased brain vascular permeability, microvascular protein leakage, and alterations of aquaporins allows for metabolites and toxins that are normally impermeable to the BBB to injure the brain and result in cerebral edema commonly seen in AKI patients [48, 49, 54, 55]. Clinical evidence of BBB disruption serving a pathophysiologic role in delirium is provided in one study that showed elevated serum levels of S100β, the marker of BBB damage [56] in elderly patients with delirium [57].
The pro-inflammatory cytokines, IL-6, TNF-α, IL-1α, IL-1β, have been implicated in delirium-like behavioral changes, such as impaired concentration, diminished motivation, and psychomotor retardation in critically ill patients [52, 58,59,60]. Among various cytokines, IL-6 has been frequently studied as a potential predictor of delirium in urinary tract infection, sepsis, acute lung injury, and perioperative animal models [53, 61,62,63,64,65,66]. Indeed, animal studies have revealed that IL-6 is both necessary and sufficient to produce cognitive decline [67]. It is postulated that surgical intervention may induce neuroinflammation and contribute to cognitive decline. For instance, it has been found that orthopedic surgery disrupts the BBB and promotes infiltration of bone marrow-derived monocytes [68] and activation of microglia [69] in rodents. This is concordant with clinical studies that have found that high levels of IL-6 preoperatively were significantly associated with postoperative delirium in patients admitted for elective and emergency surgery [62, 70]. Given that these pro-inflammatory cytokines, especially IL-6, have also been demonstrated to be elevated in AKI, similar mechanisms for delirium in AKI are likely, but remain to be proven.
Animal models demonstrate that the structural areas of the brain disproportionately affected by AKI-induced inflammatory mediators include the CA1 region of the hippocampus [13], which is consistent with the semiology of delirium and the hippocampus’ established involvement in learning and memory as well as anxiety and depression [71]. Furthermore, the CA1 neurons of the hippocampus are vulnerable to damage in several other conditions that result in neurodegeneration, including global cerebral ischemia, Alzheimer’s disease, and prion diseases [72,73,74].
Mouse models of AKI [13], prion disorders [75], and Alzheimer’s disease [76] all suggest that hippocampal CA1 pathology is in part, accountable for hypoactivity in mice. Hippocampal injury and inflammation highlighted by pyknotic neuronal cells [77], activation of microglial cells [13], upregulation of toll-like receptor-4 [78], increased levels of keratinocyte-derived chemokine, and increased levels of granulocyte colony-stimulating factor [79] within the hippocampus of renal ischemia reperfusion injury-induced AKI mouse models provide further evidence for a direct pathological role for AKI in delirium. Other areas of the brain involved include the cerebral cortex and the corpus callosum as evidenced by astrogliosis [13], a marker for activated glial cells during brain inflammation [80, 81]. Thus, the activation of central immune cells leading to neuronal injury and dysfunction [82] may contribute to post-AKI delirium (Fig. 2).
Cerebral injury from AKI may potentially be reversible if mild in severity or if treated early, as is the case with the clinical course of delirium. This point was suggested by findings by Liu et al. who found no neuronal apoptotic changes in mice with AKI as evidenced by minimal terminal deoxynucleotidyl transferase-mediated digoxigenin-deoxyuridine nick-end labeling staining and minimal caspase-3 signaling on immunostaining [13].
A summary of experimental studies on brain effects of inflammation (Table 2) is shown below.
AKI-associated fluid overload
Another potential mechanism of AKI-associated delirium is fluid overload, which can occur in 40% of patients in the ICU [83]. Fluid overload is thought to increase capillary transmural hydrostatic pressure, resulting in fluid leak into brain interstitial tissue causing cerebral edema [83]. Using a multivariate proportion odds logistic regression model for delirium in mechanically ventilated patients, a retrospective observational cohort study found that fluid overloaded patients, defined as when the recorded body weight was 10% higher than at baseline, resulted in more delirium days (OR 2.16, 95% CI 1.05–4.47) [83].
Concordantly, a study by Nguyen et al. found that fluid overload was independently associated with development of delirium in patients with shock (171 ± 104 in the delirium group vs. 128 ± 80 ml/kg; both p = 0.001) [29]. Interestingly, this study did not find a difference in central venous pressure between the two groups, suggesting that delirium due to fluid overload is independent of venous congestion; however, central venous pressure is associated with increased risk of AKI [84]. Nguyen et al. hypothesized that mechanism behind delirium from fluid overload was from brain vasogenic edema due to BBB leakage as evidenced by increased serum S100β [29], which is an early marker of BBB disruption [85]. BBB leakage thus promotes brain edema and allows for movement of neurotoxic substances into the brain [86], resulting in delirium. Thus, a reasonable approach to fluid status in a patient with AKI is to avoid hypervolemia, which not only protects the kidneys from further injury [87], but also protects the brain. Further investigations into the cognitive effects of fluid overload in the setting of AKI are warranted.
Hormonal and neurotransmitter effects of AKI
AKI may also lead to changes in the hormonal balance and neurotransmitter turnover in the brain which may contribute to delirium. Adachi et al. studied changes in the monoamine metabolism and motor activity in AKI rats [30] and found an overall decrease in dopamine turnover in the striatum, mesencephalon, and hypothalamus of AKI rats, while the turnover of norepinephrine or 5-hydroxyindoleacetic acid, the main metabolite of serotonin, was not affected by AKI. The authors postulated that the impairment of spontaneous motor activity in the AKI rats, a sign of delirium, may be related to depressed central dopamine turnover [30], resulting in impairments with memory, learning, anxiety, and depression [88,89,90].
Additionally, neurologic abnormalities from AKI may be related to the rise of calcium content in the brain [28]. This hypothesis comes from studies of AKI in canines, which identified biochemical alternations in the brain, whereby calcium contents in gray and white matter markedly increased 3 days after the onset of AKI along with a modest increment of magnesium in delirium-relevant regions of the brain. While these increases were thought to be related to excess parathyroid hormone [28], it is known that hypercalcemia is one of the reversible metabolic causes of delirium in patients with advanced cancer for instance, and that treatment of hypercalcemia resulted in symptom control [91]. Thus, for patients with delirium in the setting of AKI, it is reasonable to evaluate and treat hypercalcemia as a contributing factor.
Potential therapeutic treatments of AKI-associated delirium
There is a pressing need for novel clinical interventions to ameliorate AKI-associated delirium. With the possible exception of renal replacement therapy, existing treatment paradigms are limited to lower, indirect evidence of benefit. A summary of the potential therapeutic approaches to AKI-associated delirium is provided in Table 3.
Renal replacement therapy
A prospective observational cohort study found that renal replacement therapy modifies the risk of AKI-associated delirium. Specifically, among patients not on renal replacement therapy, an increase in daily peak serum creatinine of 1 mg/dl was significantly associated with increased odds of delirium (OR, 1.35; 95% CI, 1.18–1.55), whereas patients receiving renal replacement therapy, daily peak serum creatinine was not associated with delirium (OR, 1.07; 95% CI, 0.87–1.31). The authors hypothesized that renal replacement therapy diminishes the effects of AKI on the brain by clearing neurotoxic sedatives, antibiotics, and metabolites [2]. Although early initiation of renal replacement therapy may shorten length of stay in the intensive care [92] or in-hospital settings [93], it is unclear if improved delirium outcomes drive this effect. Prior randomized clinical trials were not designed to assess delirium as a primary outcome, leaving opportunities for future clinical trials to evaluate the potential for both benefits and risks of invasive renal replacement therapy [92, 94].
Ultrafiltration may be an intuitive approach to addressing AKI-associated delirium, due to fluid overload; however, any potential benefits of ultrafiltration need to be balanced with the concerns related to cerebral hypoperfusion [95] and worsened renal recovery [96]. Although delirium has not been specifically evaluated in prior studies using ultrafiltration, prior studies have shown improved rates of extracerebral organ dysfunction with lower compared to higher rates of ultrafiltration [96]. Future studies are needed to examine the viability of ultrafiltration in hypervolemic patients with AKI to mitigate delirium.
Additionally, data from the United States Renal Data System found the risk of incident dementia to be lower in patients on peritoneal dialysis compared to hemodialysis, with a hazard ratio 0.74 (95% CI 0.64–0.86) in a matched model. Cognitive impairment rates for patients undergoing hemodialysis are 1.5–2.0 times higher than for those undergoing peritoneal dialysis [97]. This was suggested to be attributable to a reduction in the hemodynamic instability and rapid changes in cerebral blood flow typically seen with patients undergoing hemodialysis [90]. Future studies are needed to assess whether renal replacement therapy strategies that lower the risk of hemodynamic instability potentially mitigate the risk of delirium.
Analgosedation optimization
Sedatives, such as benzodiazepines and opiates, are known to be highly deliriogenic and may potentially exacerbate delirium in the context of AKI. Careful consideration of their respective metabolism and clearance pathways is warranted when choosing between various sedatives in the setting of AKI.
Metabolites of commonly used benzodiazepines, for example, the active midazolam metabolite, α-hydroxymidazolam, are cleared by the kidneys and accumulate with renal failure [98], which may prolong the duration of their pharmacological effects [23]. In contrast, the lorazepam metabolite, lorazepam glucuronide, is a nontoxic metabolite and its drug clearance is not altered by renal disease [99]. As such, studies from patients with end-stage renal disease report lower risk of accumulation with lorazepam compared to midazolam, diazepam, clonazepam [100, 101]. Despite the relative renal safety of lorazepam, a side effect of the solvent used in intravenous lorazepam can produce propylene glycol toxicity, which can lead to proximal tubular necrosis resulting in AKI, in addition to metabolic acidosis, serum hyperosmolality, and elevated anion gap [102]. Patients with AKI may be at higher risk of propylene glycol accumulation, although it is reassuring that while in one study, propylene glycol toxicity occurred in 19% of medical ICU patients receiving high-dose lorazepam infusion, none had any significant clinical deterioration [103].
Similar considerations related to the mechanism of drug elimination are justified when using opioids in patients with AKI. Opioids that undergo significant clearance through the kidneys should be avoided. For instance, the metabolites of tramadol, morphine, codeine, and meperidine are renally cleared [45, 104] and thus justify caution or dose adjustment in the setting of AKI. In particular, the morphine metabolite, morphine-6-glucuronide, has prolonged effects in the brain once it crosses the BBB, and even with discontinuation or dialyzing to remove the metabolite, the brain effects persist for some time as morphine-6-glucuronide re-equilibrates across the BBB [105]. Additionally, a systematic review by Swart et al. on the comparative risk of delirium with different opioids found the highest risk of delirium with meperidine and tramadol, likely due to the high anticholinergic properties of the opioids and their metabolites [44].
In contrast, fentanyl is extensively metabolized via CYP3A4 into norfentanyl, which is an inactive metabolite, and methadone is ultimately metabolized into pyrroline, which can be eliminated through feces [104]. The risk of delirium was noted to be lower with fentanyl compared to other opioids [44], findings similar to that of a systematic review by King et al. which noted less harm in renally impaired patients when using fentanyl compared to other opioids, such as morphine [106]. In fact, fentanyl use has been associated with reduced delirium [83]; thus, in patients with AKI, given the safety profile with renal dysfunction as well as relatively low risk of delirium, fentanyl should be the first-line opioid when indicated.
Neuropathic agents, such as tricyclic antidepressants, are known contributors to delirium—perhaps mediated by their anticholinergic effects [107]. Thus, such agents must be used with caution in patients with renal disease given that serum levels of glucuronidated metabolites of tricyclic antidepressants accumulate with kidney dysfunction. Other neuropathic agents such as gabapentin and pregabalin must also be used cautiously in the setting of AKI since these agents are renally excreted. The toxic accumulation of such drugs may result in depressed mental status [108] and mimic delirium.
Dexmedetomidine, an α-2 adrenergic agonist, may be superior to other sedatives such as benzodiazepines, propofol, and opioids [109,110,111] given its lack of ϒ-aminobutyric acid properties, as with benzodiazepines and propofol, or anticholinergic properties, such as with opioids, both of which are thought to play a role in the pathogenesis of delirium. Dexmedetomidine may also provide a more natural sleep-like sedation pattern, which may reduce the risk of develo** delirium [110]. Dexmedetomidine’s delirium-sparing effects in AKI may also be related to its hepatic clearance [112], and potential renoprotective effects [113] via stabilization of the sympathetic system, and anti-inflammatory toll-like receptor-4-mediated effects [113]. Therefore, in the setting of AKI, it seems reasonable to use dexmedetomidine over other types of sedatives [114].
Potential emerging therapies and future investigations
In the future, immunomodulating therapies may play a role in the prevention and treatment of AKI-associated delirium. Although various cytokines and inflammatory mediators are upregulated in AKI, recent animal studies suggest that the use of systemic IL-6 inhibition mitigates delirium-like phenotypes in urinary tract infection [66], acute lung injury [65], and postoperative states [67]. Given that IL-6 is significantly upregulated in AKI [115], future studies are needed to determine whether modulation of the IL-6 signaling pathway mitigates AKI-associated delirium.
As noted earlier, uremic guanidino compounds may play a central role in AKI-associated delirium by promoting NMDA receptor-mediated depolarization of hippocampal neurons, therefore minimizing epileptiform discharges, and hippocampal damage [33]. In a study using rodents, ketamine, an NMDA receptor antagonist, prevented epileptiform activity and hippocampal damage induced by injection of guanidinosuccinic acid, one of the key neuroanatomical structures believed to be affected in AKI-associated delirium [116]. Therefore, future studies are needed to assess the role of NMDA receptor antagonists to prevent or treat AKI-associated delirium. While studies using NMDA receptor antagonists to reduce postoperative delirium have yielded mixed results, further research is needed to assess the role of NMDA receptor agonists specifically in AKI-associated delirium.
Conclusion
The pathogenesis of AKI-associated delirium is multifactorial and includes both inflammatory- and non-inflammatory-mediated processes, such as the accumulation of toxins and drugs, structural brain injury from systemic inflammation, impaired volume homeostasis, and hormonal and neurotransmitter effects. Current evidence suggests that, when possible, gradual normalization of kidney function may ameliorate delirium; however, optimization of other clinical contributors, such as deliriogenic drugs, including analgosedation and antibiotics, provides additional opportunities to mitigate delirium. Future investigations are needed to understand the role of systemic immunomodulation to ameliorate AKI-associated delirium.
Availability of data and materials
Data in this study were a review of existing data, which are openly available at locations cited in the reference section.
References
Jäckel M, Aicher N, Rilinger J, et al. Incidence and predictors of delirium on the intensive care unit in patients with acute kidney injury, insight from a retrospective registry. Sci Rep. 2021;11:17260. https://doi.org/10.1038/s41598-021-96839-x.
Siew ED, Fissell WH, Tripp CM, et al. Acute kidney injury as a risk factor for delirium and coma during critical illness. Am J Respir Crit Care Med. 2016;195(12):1597–607.
Jayaswal AK, Sampath H, Soohinda G, Dutta S. Delirium in medical intensive care units: Incidence, subtypes, risk factors, and outcome. Indian J Psychiatry. 2019;61(4):352–8. https://doi.org/10.4103/psychiatry.IndianJPsychiatry_583_18.
Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–23.
Zipser CM, Deuel J, Ernst J, et al. Predisposing and precipitating factors for delirium in neurology: a prospective cohort study of 1487 patients. J Neurol. 2019;266(12):3065–75. https://doi.org/10.1007/s00415-019-09533-4.
Kotfis K, Marra A, Ely EW. ICU delirium: a diagnostic and therapeutic challenge in the intensive care unit. Anaesthesiol Intensive Ther. 2018;50(2):160–7. https://doi.org/10.5603/AIT.a2018.0011.
Salluh JI, Soares M, Teles JM, Delirium Epidemiology in Critical Care Study Group, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care. 2010;14(6):R210. https://doi.org/10.1186/cc9333.
Kellum JA, Murugan R. Effects of non-severe acute kidney injury on clinical outcomes in critically ill patients. Crit Care. 2016;20:159. https://doi.org/10.1186/s13054-016-1295-4.
Rizo-Topete LM, Rosner MH, Ronco C. Acute kidney injury risk assessment and the nephrology rapid response team. Blood Purif. 2017;43(1–3):82–8. https://doi.org/10.1159/000452402.
Hoke TS, Douglas IS, Klein CL, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol JASN. 2007;18(1):155–64. https://doi.org/10.1681/ASN.2006050494.
Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14(6):1549–58. https://doi.org/10.1097/01.ASN.0000064946.94590.46.
Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37(2):85–98.
Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol JASN. 2008;19(7):1360–70. https://doi.org/10.1681/ASN.2007080901.
Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg. 2004;107:1–16.
Grams ME, Rabb H. The distant organ effects of acute kidney injury. Kidney Int. 2012;81:942–8.
Tsai HH, Yen RF, Lin CL, Kao CH. Increased risk of dementia in patients hospitalized with acute kidney injury: a nationwide population-based cohort study. PLoS ONE. 2017;12(2):e0171671. https://doi.org/10.1371/journal.pone.0171671.
Guerra C, Linde-Zwirble WT, Wunsch H. Risk factors for dementia after critical illness in elderly Medicare beneficiaries. Crit Care. 2012;16:R233.
Doi K, Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int. 2016;89(3):555–64. https://doi.org/10.1016/j.kint.2015.11.019.
Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol. 2020;77(11):1373–81. https://doi.org/10.1001/jamaneurol.2020.2273.
Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16. https://doi.org/10.1056/NEJMoa1301372.
Hobson VL, Hall JR, Humphreys-Clark JD, Schrimsher GW, O’Bryant SE. Identifying functional impairment with scores from the repeatable battery for the assessment of neuropsychological status (RBANS). Int J Geriatr Psychiatry. 2010;25(5):525–30. https://doi.org/10.1002/gps.2382.
Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly. Arch Intern Med. 2008;168(1):27–32. https://doi.org/10.1001/archinternmed.2007.4.
Siew ED, Fissell WH, Tripp CM, et al. Acute kidney injury as a risk factor for delirium and coma during critical illness. Am J Respir Crit Care Med. 2017;195(12):1597–607. https://doi.org/10.1164/rccm.201603-0476OC.
Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167:1629–34.
Wan R, McKenzie CA, Taylor D, Camporota L, Ostermann M. Acute kidney injury as a risk factor of hyperactive delirium: a case control study. J Crit Care. 2020;55:194–7. https://doi.org/10.1016/j.jcrc.2019.10.013.
Hosker C, Ward D. Hypoactive delirium. BMJ. 2017. https://doi.org/10.1136/bmj.j2047.
Lee SA, Cozzi M, Bush EL, Rabb H. Distant organ dysfunction in acute kidney injury: a review. Am J Kidney Dis Off J Natl Kidney Found. 2018;72(6):846–56. https://doi.org/10.1053/j.ajkd.2018.03.028.
Arieff AI, Massry SG. Calcium metabolism of brain in acute renal failure. Effects of uremia, hemodialysis, and parathyroid hormone. J Clin Invest. 1974;53(2):387–92.
Nguyen DN, Huyghens L, Parra J, Schiettecatte J, Smitz J, Vincen JL. Hypotension and a positive fluid balance are associated with delirium in patients with shock. PLoS ONE. 2018;13:e0200495.
Adachi N, Lei B, Deshpande G, et al. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive Care Med. 2001;27(10):1655–60. https://doi.org/10.1007/s001340101067.
Olano CG, Akram SM, Bhatt H. Uremic encephalopathy. In: StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK564327/. Accessed 21 June 2022.
Popkov VA, Silachev DN, Zalevsky AO, Zorov DB, Plotnikov EY. Mitochondria as a source and a target for uremic toxins. Int J Mol Sci. 2019;20(12):E3094. https://doi.org/10.3390/ijms20123094.
Deyn PPD, D’Hooge R, Bogaert PPV, Marescau B. Endogenous guanidino compounds as uremic neurotoxins. Kidney Int. 2001;59:S77–83. https://doi.org/10.1046/j.1523-1755.2001.59780077.x.
Tanaka S, Okusa MD. Cross-talk between the nervous system and the kidney. Kidney Int. 2020;97(3):466–76. https://doi.org/10.1016/j.kint.2019.10.032.
Dhooge R, Pei YQ, Marescau B, De Deyn PP. Convulsive action and toxicity of uremic guanidino compounds: behavioral assessment and relation to brain concentration in adult mice. J Neurol Sci. 1992;112(1):96–105. https://doi.org/10.1016/0022-510X(92)90138-B.
Gungor O, Aydin Z, Inci A, Oguz EG, Arici M. Seizures in patients with kidney diseases: a neglected problem? Nephrol Dial Transplant. 2021. https://doi.org/10.1093/ndt/gfab283.
Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit*. Crit Care Med. 2009;37(6):2051–6. https://doi.org/10.1097/CCM.0b013e3181a00604.
Grill MF, Maganti R. Cephalosporin-induced neurotoxicity: clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann Pharmacother. 2008;42(12):1843–50. https://doi.org/10.1345/aph.1L307.
Sonck J, Laureys G, Verbeelen D. The neurotoxicity and safety of treatment with cefepime in patients with renal failure. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc. 2008;23(3):966–70. https://doi.org/10.1093/ndt/gfm713.
Shea YF, Mok MY, Cheng KC, Hon FK, Chu LW. Delayed recovery from ertapenem induced encephalopathy: case-report and a possible mechanism. Int J Clin Pharm. 2013;35(4):535–7. https://doi.org/10.1007/s11096-013-9812-x.
Bhattacharyya S, Darby RR, Raibagkar P, Gonzalez Castro LN, Berkowitz AL. Antibiotic-associated encephalopathy. Neurology. 2016;86(10):963–71. https://doi.org/10.1212/WNL.0000000000002455.
Payne LE, Gagnon DJ, Riker RR, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care. 2017;21:276. https://doi.org/10.1186/s13054-017-1856-1.
Vutukuri R, Brunkhorst R, Kestner RI, et al. Alteration of sphingolipid metabolism as a putative mechanism underlying LPS-induced BBB disruption. J Neurochem. 2018;144:172–85. https://doi.org/10.1111/jnc.14236.
Swart LM, van der Zanden V, Spies PE, de Rooij SE, van Munster BC. The comparative risk of delirium with different opioids: a systematic review. Drugs Aging. 2017;34(6):437–43. https://doi.org/10.1007/s40266-017-0455-9.
Simopoulos TT, Smith HS, Peeters-Asdourian C, Stevens DS. Use of meperidine in patient-controlled analgesia and the development of a normeperidine toxic reaction. Arch Surg. 2002;137(1):84–8. https://doi.org/10.1001/archsurg.137.1.84.
Szeto HH, Inturrisi CE, Houde R, Saal S, Cheigh J, Reidenberg MM. Accumulation of normeperidine, an active metabolite of meperidine, in patients with renal failure or cancer. Ann Intern Med. 1977;86(6):738–41. https://doi.org/10.7326/0003-4819-86-6-738.
MacLullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65(3):229–38. https://doi.org/10.1016/j.jpsychores.2008.05.019.
Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology. 2012;116:1139–48.
Wan RYY, Ostermann M. Acute kidney injury and delirium: kidney–brain crosstalk. In: Vincent JL, editors. Annual update in intensive care and emergency medicine. Springer; 2019. p. 397–404. https://doi.org/10.1007/978-3-030-06067-1_31
Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–58.
Kramer AA, Postler G, Salhab KF, et al. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55:2362–7.
Park SW, Chen SW, Kim M, et al. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest. 2011;91:63–84.
Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol (Berl). 2010;119(6):737–54. https://doi.org/10.1007/s00401-010-0674-1.
Hariri RJ. Cerebral edema. Neurosurg Clin N Am. 1994;5(4):687–706.
de Castro RM, Hirt L, Bogousslavsky J, Regli L, Badaut J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J Neurosci Res. 2006;83(7):1231–40. https://doi.org/10.1002/jnr.20819.
Marchi N, Cavaglia M, Fazio V, Bhudia S, Hallene K, Janigro D. Peripheral markers of blood-brain barrier damage. Clin Chim Acta Int J Clin Chem. 2004;342(1–2):1–12. https://doi.org/10.1016/j.cccn.2003.12.008.
van Munster BC, Korevaar JC, Korse CM, Bonfrer JM, Zwinderman AH, de Rooij SE. Serum S100B in elderly patients with and without delirium. Int J Geriatr Psychiatry. 2010;25(3):234–9. https://doi.org/10.1002/gps.2326.
Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352. https://doi.org/10.1186/s13054-019-2626-z.
Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68(3):360–8. https://doi.org/10.1002/ana.22082.
Barrientos RM, Higgins EA, Biedenkapp JC, et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–32. https://doi.org/10.1016/j.neurobiolaging.2005.03.010.
Androsova G, Krause R, Winterer G, Schneider R. Biomarkers of postoperative delirium and cognitive dysfunction. Front Aging Neurosci. 2015;7:112. https://doi.org/10.3389/fnagi.2015.00112.
Capri M, Yani SL, Chattat R, et al. Pre-operative, high-IL-6 blood level is a risk factor of post-operative delirium onset in old patients. Front Endocrinol. 2014;5:173. https://doi.org/10.3389/fendo.2014.00173.
van Munster BC, Zwinderman AH, de Rooij SE. Genetic variations in the interleukin-6 and interleukin-8 genes and the interleukin-6 receptor gene in delirium. Rejuvenation Res. 2011;14(4):425–8. https://doi.org/10.1089/rej.2011.1155.
Vasunilashorn SM, Ngo L, Inouye SK, et al. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J Gerontol A Biol Sci Med Sci. 2015;70(10):1289–95. https://doi.org/10.1093/gerona/glv083.
Sparrow NA, Anwar F, Covarrubias AE, et al. IL-6 inhibition reduces neuronal injury in a murine model of ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2021;65(4):403–12. https://doi.org/10.1165/rcmb.2021-0072OC.
Rashid MH, Sparrow NA, Anwar F, et al. Interleukin-6 mediates delirium-like phenotypes in a murine model of urinary tract infection. J Neuroinflamm. 2021;18(1):247. https://doi.org/10.1186/s12974-021-02304-x.
Hu J, Feng X, Valdearcos M, et al. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. BJA Br J Anaesth. 2018;120(3):537–45. https://doi.org/10.1016/j.bja.2017.11.096.
Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986–95. https://doi.org/10.1002/ana.22664.
Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2(7):e91229. https://doi.org/10.1172/jci.insight.91229.
Chen Y, Lu S, Wu Y, et al. Change in serum level of interleukin 6 and delirium after coronary artery bypass graft. Am J Crit Care. 2019;28(6):462–70. https://doi.org/10.4037/ajcc2019976.
Heldt S, Stanek L, Chhatwal J, Ressler K. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12(7):656–70. https://doi.org/10.1038/sj.mp.4001957.
White BC, Grossman LI, O’Neil BJ, et al. Global brain ischemia and reperfusion. Ann Emerg Med. 1996;27(5):588–94. https://doi.org/10.1016/s0196-0644(96)70161-0.
White BC, Sullivan JM, DeGracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179(1–2):1–33. https://doi.org/10.1016/s0022-510x(00)00386-5.
Giannakopoulos P, Herrmann FR, Bussière T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60(9):1495–500. https://doi.org/10.1212/01.wnl.0000063311.58879.01.
Cunningham C, Deacon RMJ, Chan K, Boche D, Rawlins JNP, Perry VH. Neuropathologically distinct prion strains give rise to similar temporal profiles of behavioral deficits. Neurobiol Dis. 2005;18(2):258–69. https://doi.org/10.1016/j.nbd.2004.08.015.
Nelson RL, Guo Z, Halagappa VM, et al. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp Neurol. 2007;205(1):166–76. https://doi.org/10.1016/j.expneurol.2007.01.037.
Chou AH, Lee CM, Chen CY, et al. Hippocampal transcriptional dysregulation after renal ischemia and reperfusion. Brain Res. 2014;1582:197–210. https://doi.org/10.1016/j.brainres.2014.07.030.
Salama M, Mohamed Farrag S, Abulfath Abulasrar S, et al. Up-regulation of TLR-4 in the brain after ischemic kidney-induced encephalopathy in the rat. CNS Neurol Disord Drug Targets. 2013;12(5):583–6.
Molls RR, Savransky V, Liu M, et al. Keratinocyte-derived chemokine is an early biomarker of ischemic acute kidney injury. Am J Physiol Renal Physiol. 2006;290(5):F1187-1193. https://doi.org/10.1152/ajprenal.00342.2005.
Trzepacz PT. The neuropathogenesis of delirium. A need to focus our research. Psychosomatics. 1994;35(4):374–91. https://doi.org/10.1016/S0033-3182(94)71759-X.
Ali S, Patel M, Jabeen S, et al. Insight into delirium. Innov Clin Neurosci. 2011;8(10):25–34.
Yanuck SF. Microglial phagocytosis of neurons: diminishing neuronal loss in traumatic, infectious, inflammatory, and autoimmune CNS disorders. Front Psychiatry. 2019;10:712. https://doi.org/10.3389/fpsyt.2019.00712.
Ouchi A, Sakuramoto H, Hoshino H, et al. Association between fluid overload and delirium/coma in mechanically ventilated patients. Acute Med Surg. 2020;7(1):e508. https://doi.org/10.1002/ams2.508.
Legrand M, Dupuis C, Simon C, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care Lond Engl. 2013;17(6):R278. https://doi.org/10.1186/cc13133.
Kapural M, Krizanac-Bengez L, Barnett G, et al. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Res. 2002;940(1–2):102–4. https://doi.org/10.1016/s0006-8993(02)02586-6.
Davies DC. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J Anat. 2002;200(6):639–46. https://doi.org/10.1046/j.1469-7580.2002.00065.x.
Zhang J, Crichton S, Dixon A, Seylanova N, Peng ZY, Ostermann M. Cumulative fluid accumulation is associated with the development of acute kidney injury and non-recovery of renal function: a retrospective analysis. Crit Care. 2019;23(1):392. https://doi.org/10.1186/s13054-019-2673-5.
Brown AS, Gershon S. Dopamine and depression. J Neural Transm Gen Sect JNT. 1993;91(2):75–109. https://doi.org/10.1007/BF01245227.
Gjerris A, Werdelin L, Rafaelsen OJ, Alling C, Christensen NJ. CSF dopamine increased in depression: CSF dopamine, noradrenaline and their metabolites in depressed patients and in controls. J Affect Disord. 1987;13(3):279–86. https://doi.org/10.1016/0165-0327(87)90048-6.
Lu R, Kiernan MC, Murray A, Rosner MH, Ronco C. Kidney–brain crosstalk in the acute and chronic setting. Nat Rev Nephrol. 2015;11:707–19.
Delgado-Guay MO, Yennurajalingam S, Bruera E. Delirium with severe symptom expression related to hypercalcemia in a patient with advanced cancer: an interdisciplinary approach to treatment. J Pain Symptom Manag. 2008;36(4):442–9. https://doi.org/10.1016/j.jpainsymman.2007.11.004.
Bagshaw SM, Wald R, Adhikari NK, Bellomo R, da Costa BR, Dreyfuss D, Gallagher MP, Gaudry S, Hoste E, Lamontagne F, Joannidis M. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383(3):240–51. https://doi.org/10.1056/NEJMoa2000741.
Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190–9. https://doi.org/10.1001/jama.2016.5828.
Li X, Liu C, Mao Z, Li Q, Zhou F. Timing of renal replacement therapy initiation for acute kidney injury in critically ill patients: a systematic review of randomized clinical trials with meta-analysis and trial sequential analysis. Crit Care. 2021;25(1):15. https://doi.org/10.1186/s13054-020-03451-y.
Eldehni MT, McIntyre CW. Are there neurological consequences of recurrent intradialytic hypotension? Semin Dial. 2012;25(3):253–6. https://doi.org/10.1111/j.1525-139X.2012.01057.x.
Murugan R, Kerti SJ, Chang CCH, et al. Association between net ultrafiltration rate and renal recovery among critically ill adults with acute kidney injury receiving continuous renal replacement therapy: an observational cohort study. Blood Purif. 2021. https://doi.org/10.1159/000517281.
Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2004 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis Off J Natl Kidney Found. 2005;45(1 Suppl 1):A5–7. https://doi.org/10.1053/j.ajkd.2004.10.009.
Prommer E. Midazolam: an essential palliative care drug. Palliat Care Soc Pract. 2020;14:2632352419895527. https://doi.org/10.1177/2632352419895527.
Morrison G, Chiang ST, Koepke HH, Walker BR. Effect of renal impairment and hemodialysis on lorazepam kinetics. Clin Pharmacol Ther. 1984;35(5):646–52. https://doi.org/10.1038/clpt.1984.89.
Wilcock A, Charlesworth S, Twycross R, et al. Prescribing non-opioid drugs in end-stage kidney disease. J Pain Symptom Manag. 2017;54(5):776–87. https://doi.org/10.1016/j.jpainsymman.2017.08.014.
Arulkumaran N, Montero RM, Singer M. Management of the dialysis patient in general intensive care. Br J Anaesth. 2012;108(2):183–92. https://doi.org/10.1093/bja/aer461.
Zar T, Graeber C, Perazella MA. Reviews: Recognition, treatment, and prevention of propylene glycol toxicity. Semin Dial. 2007;20(3):217–9. https://doi.org/10.1111/j.1525-139X.2007.00280.x.
Wilson KC, Reardon C, Theodore AC, Farber HW. Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: a case series and prospective, observational pilot study. Chest. 2005;128(3):1674–81. https://doi.org/10.1378/chest.128.3.1674.
Florida SG PharmD, BCPS Assistant Professor University of South Florida College of Pharmacy Department of Pharmacotherapeutics and Clinical Research Tampa, Florida Engi Nakhla, PharmD, CPh Clinical Pharmacist Tampa General Hospital Tampa. Opioid dosing in renal and hepatic impairment. https://www.uspharmacist.com/article/opioid-dosing-in-renal-and-hepatic-impairment. Accessed 26 Jan 2022.
Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manag. 2004;28(5):497–504. https://doi.org/10.1016/j.jpainsymman.2004.02.021.
King S, Forbes K, Hanks G, Ferro C, Chambers E. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliat Med. 2011;25(5):525–52. https://doi.org/10.1177/0269216311406313.
By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. https://doi.org/10.1111/jgs.15767.
Miller A, Price G. Gabapentin toxicity in renal failure: the importance of dose adjustment. Pain Med. 2009;10(1):190–2. https://doi.org/10.1111/j.1526-4637.2008.00492.x.
Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–99. https://doi.org/10.1001/jama.2009.56.
Flükiger J, Hollinger A, Speich B, et al. Dexmedetomidine in prevention and treatment of postoperative and intensive care unit delirium: a systematic review and meta-analysis. Ann Intensive Care. 2018;8(1):92. https://doi.org/10.1186/s13613-018-0437-z.
Pereira JV, Sanjanwala RM, Mohammed MK, Le ML, Arora RC. Dexmedetomidine versus propofol sedation in reducing delirium among older adults in the ICU: a systematic review and meta-analysis. Eur J Anaesthesiol. 2020;37(2):121–31. https://doi.org/10.1097/EJA.0000000000001131.
Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. https://doi.org/10.1007/s40262-017-0507-7.
Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 2018;18:7. https://doi.org/10.1186/s12871-018-0472-1.
Strøm T, Johansen RR, Prahl JO, Toft P. Sedation and renal impairment in critically ill patients: a post hoc analysis of a randomized trial. Crit Care. 2011;15(3):R119. https://doi.org/10.1186/cc10218.
Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol JASN. 2008;19(6):1106–15. https://doi.org/10.1681/ASN.2007070744.
Pan JC, Pei YQ, An L, Lai L, D’Hooge R, De Deyn PP. Epileptiform activity and hippocampal damage produced by intrahippocampal injection of guanidinosuccinic acid in rat. Neurosci Lett. 1996;209(2):121–24. https://doi.org/10.1016/0304-3940(96)12615-X
Lieberman JA, Cooper TB, Suckow RF, et al. Tricyclic antidepressant and metabolite levels in chronic renal failure. Clin Pharmacol Ther. 1985;37(3):301–7. https://doi.org/10.1038/clpt.1985.44.
Acknowledgements
We acknowledge Faizan Anwar for his contribution to graphic design for Fig. 2.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
HP drafted the manuscript. SK, WE, MG, and SL read, edited, and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author(s) declared no potential competing interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pang, H., Kumar, S., Ely, E.W. et al. Acute kidney injury-associated delirium: a review of clinical and pathophysiological mechanisms. Crit Care 26, 258 (2022). https://doi.org/10.1186/s13054-022-04131-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04131-9