Abstract
In women who are getting older, the quantity and quality of their follicles or oocytes and decline. This is characterized by decreased ovarian reserve function (DOR), fewer remaining oocytes, and lower quality oocytes. As more women choose to delay childbirth, the decline in fertility associated with age has become a significant concern for modern women. The decline in oocyte quality is a key indicator of ovarian aging. Many studies suggest that age-related changes in oocyte energy metabolism may impact oocyte quality. Changes in oocyte energy metabolism affect adenosine 5'-triphosphate (ATP) production, but how related products and proteins influence oocyte quality remains largely unknown. This review focuses on oocyte metabolism in age-related ovarian aging and its potential impact on oocyte quality, as well as therapeutic strategies that may partially influence oocyte metabolism. This research aims to enhance our understanding of age-related changes in oocyte energy metabolism, and the identification of biomarkers and treatment methods.
Similar content being viewed by others
Introduction
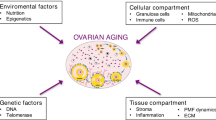
Ovarian aging is a significant cause of female infertility [1]. As a woman ages, the quantity and quality of the follicle or oocyte degenerates, resulting in a decrease in ovarian reserve function (DOR). During this process, fewer oocytes are produced in the ovaries and their quality or ability is diminished. Menopause is the last stage of the ovarian aging process, with most women entering menopause between the ages of 49 and 52. [2]. In modern society, women often postpone childbirth due to a variety of factors, including economics, careers, and lifestyles [3]. However, as humans age, fertility rates begin to decline around 30 years of age and become clinically relevant between the ages of 35 and 40, after which they continue to decline significantly. [4]. The decline in fertility associated with women’s age has become an important issue that troubles modern women.
Changes in the energy metabolism of oocytes due to age can affect the cellular levels of intermediates and byproducts, consequently impacting oocyte quality. However, the mechanisms underlying the effects of changes in the intermediary steps of energy metabolism on adenosine 5'-triphosphate (ATP) generation in oocytes, as well as the influence of related products and proteins on oocyte quality and subsequent ovarian aging, remain unclear.
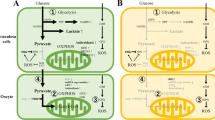
The metabolism of energy is important in the development and maturation of oocytes. Energy metabolism processes influence nutrient absorption, macromolecular biosynthesis, energy production, and cellular redox status. The mitochondria-nucleus communication plays a critical role in cellular adaptability, organismal health, and longevity, as well as energy metabolism. [5, 6]. The metabolic pathways of oocytes are complex (Fig. 1). Although cumulus cells produce ATP and provide it to oocytes [7], a decrease in ATP, a decrease in energy production capacity, and a decline in mitochondrial function are all part of the aging of the oocytes [8,9,10,11,12,13,14,15]. Reduced ATP production leads to a decline in oocyte quality, specifically resulting in decreased metabolic activity, which may affect cell cycle regulation, spindle formation during mitosis, chromosome segregation, fertilization, embryo development, and implantation, as discussed in other literature [16,17,18]. Age-related changes in oocyte energy metabolism can affect the expression of intermediates and byproducts within the cell, thereby influencing oocyte quality. However, it remains unclear how changes in intermediary steps of energy metabolism in oocytes affect ATP generation and how related products and proteins influence oocyte quality, consequently affecting ovarian aging.
Energy metabolism of oocytes. Oocyte metabolism relies on glucose metabolites provided by cumulus cells. The majority of glucose is metabolized in cumulus cells through anaerobic glycolysis, resulting in lactate production. Cumulus cells can convert glucose into pyruvate, lactate, or nicotinamide adenine dinucleotide phosphate (NADPH) through anaerobic glycolysis and the pentose phosphate pathway. These metabolites are then transferred to oocytes through paracrine signaling and gap junctions, providing energy substrates for oocyte metabolism. Oocytes generate ATP through the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS). Additionally, some glucose can be directly transported to oocytes and metabolized through the pentose phosphate pathway and hexosamine synthesis pathway. Oocytes acquire free fatty acids from the follicular fluid and gap junctions with cumulus cells, and they can also synthesize fatty acids endogenously. After entering the cells, free fatty acids can be converted and stored in lipid droplets or enter mitochondria for β-oxidation. Fatty acids in lipid droplets are esterified and stored as neutral triglycerides (TAGs). Fatty acyl-CoA is synthesized by acyl-CoA synthetases, which catalyze triglycerides into fatty acyl-CoA. Carnitine transports fatty acyl-CoA to mitochondria. The TCA cycle and OXPHOS in mitochondria process fatty acids into acetyl-CoA that is then oxidized, producing ATP once they enter the mitochondrial matrix. Glutamine enters oocytes through the follicular fluid and gap junctions, and oocytes can also synthesize glutamine. Glutamine is metabolized in the mitochondrial matrix as a fuel source for the cycle. Oocytes may possess the ability to convert Adenosine monophosphate (AMP) to ATP through the adenosine salvage pathway. Cumulus cells can also produce ATP through the adenosine salvage pathway and directly supply ATP and AMP to oocytes through gap junctions
Therefore, this review focuses on the oocyte metabolism in age-related ovarian aging and its impact on oocyte quality. This study investigates the relationship between age-related changes in oocyte energy metabolism, decline in oocyte quality, and subsequent decrease in fertility rates. In addition, it helps identify biomarkers and treatment methods.
Ovarian aging and oocyte energy metabolism
Alterations in several facets of oocyte energy metabolism in individuals suffering from ovarian senescence, such as the Tricarboxylic Acid (TCA) Cycle, Oxidative Phosphorylation, Lipid Metabolism, Glutamine Metabolism, and the Adenosine Remedial Pathway, critically impact the quality of oocytes (Fig. 2).
TCA cycle
The TCA cycle completely oxidizes the acetyl coenzyme A in cells to produce CO2, ATP, nicotinamide adenine dinucleotide (NADH), and flavin adenine dinucleotide (FADH2), and subsequently OXPHOS for the production of ATP. The TCA cycle is a crucial component in signaling pathways and metabolic disorders associated with aging, making it an important target for anti-aging treatment strategies [19, 20]. The TCA cycle takes place in oocytes, and its activity is inhibited with age [7, 11, 21]. Specifically, as age increases, the cross-regional transport of substances such as pyruvate and lactate salts from granulosa cells to oocytes decreases, but metabolites such as pyruvate, lactate salts, and glutamine gradually accumulate in the oocytes [7, 22]. Glucose [7], glucose-6-phosphate [7], sorbitol [13], mannitol [13], urea cycle intermediates such as aspartate [7], ornithine [7], and arginine [7] increase in oocytes of older mothers, indicating that energy substrates are diverted to the pentose phosphate pathway, hexosamine synthesis pathway, and urea cycle; the TCA cycle cannot process available substrates. Furthermore, the TCA cycle intermediates succinate [7], fumarate [7], citrate [11], isocitrate [11], and malate [11] decrease in an age-dependent manner in oocytes. The reduced levels of NAD + and FAD in oocytes are also observed [7, 11, 23,24,25,26]. The age-related changes in TCA cycle activity differ between species. For example, in the oocytes of horses, although the glucose abundance in the cumulus cells of older horses is higher, the level of pyruvate in the oocytes of older mares is consistently lower than that of young mares during the GV, MI, and MII stages. This suggests impaired transport or production of pyruvate, possibly due to reduced transzonal transport [13, 22]. These differences may be attributed to variations in samples and species.
The activity of TCA cycle metabolism decreases with age, leading to reduced levels of NAD + restoration [7, 23,24,25]. NAD + plays a central role in controlling hundreds of pathways in both energy metabolism and cell survival. Both NAD + and its reduced form are involved in various biological processes [20, 27,28,29,30]. Increased NAD + production or decreased degradation appears to be profitable, as reduced NAD + levels can lead to metabolic and age-related diseases [31]. In terms of aging, NAD + is essential in antioxidation, mitochondrial function, central carbon metabolism, cellular aging, protein deacetylation, and DNA damage [20, 29, 30]. Some enzymes consume NAD + , such as the sirtuin enzyme family (SIRTs) and poly(ADP-ribose) polymerase (PARP) [20]. These enzymes have become critical factors in aging [20]. In mouse ovaries with a knockout of NAD + synthesis genes, NAD + levels decrease in mid-aged mice, resulting in the impairment of oocyte quality, characterized by increased abnormal spindle and reactive oxygen species (ROS) formation [32]. Supplementation of NAD + precursor nicotinamide riboside (NR) can increase ovarian reserve and improve oocyte quality [32, 33].
The alterations in the TCA cycle intermediates can impact oocyte quality. The levels of TCA cycle intermediates, including succinic acid [7], jasmonic acid [7], citrate [11], malate [11], and fumarate [11], decrease with age in oocytes and can influence epigenetic changes. Decreased levels of succinic acid and fumarate can influence the levels of DNA and histone methylation, while decreased levels of citrate can weaken its ability to enhance histone acetylation. These effects can further contribute to the aging process [34, 35]. In addition, the TCA cycle is also related to metabolite production and biosynthesis. Intermediates of the TCA cycle can serve as precursors for amino acid synthesis, nucleotide synthesis, and fatty acid and cholesterol synthesis. In oocytes, oral administration of dimethyl fumarate can alleviate oxidative stress and delay age-related infertility in mice ovaries [36]. Moreover, the decrease in citrate levels within the follicular fluid has the potential to impact the process of oocyte maturation [37].
Phosphorylation of oxidation
The decline in oxidative phosphorylation caused by mitochondrial dysfunction is an important marker of human aging [38]. In oocytes, the energy released from glycolysis and the TCA cycle is mostly stored in reduced coenzymes and needs to be synthesized into ATP through the oxidative phosphorylation process in the mitochondria [21]. The respiratory chain consists of more than 15 components, mainly including NADH dehydrogenase (complex I), succinate dehydrogenase (complex II), cytochrome c oxidoreductase (complex III), cytochrome c oxidase (complex IV), coenzyme Q (CoQ), and cytochrome C. The electron transport chain (ETC) facilitates the translocation of protons (H +) from the matrix to the intermembrane space, consequently establishing a proton-motive force (PMF). The energy produced by PMF is used by ATP synthase to phosphorylate adenosine diphosphate (ADP) into ATP. There are two non-exclusive mechanisms for regulating oxidative phosphorylation. It can be dynamically regulated, enabling an adjustment in ATP synthesis rate to meet ATP demand [10]. Oxidative phosphorylation can also be regulated by altering the number of mitochondria [10]. OXPHOS is highly active in oocytes [39]. With age, ATP generation through oxidative phosphorylation in oocytes decreases, and the function of ETC is impaired [12,13,14]. The expression of the majority of genes encoding subunits of respiratory chain complexes I to V is downregulated [11, 23, 334,335,336,337,338].
Others
In recent years, many therapies have been proven to improve the processes related to oocyte energy metabolism. For example, brown adipose tissue-derived exosomes can prevent the deterioration of mitochondrial function caused by oocyte aging in mice and increase the ATP content in oocytes [339]. Salidroside can promote lipid metabolism, improve mitochondrial function, and enhance the maturation of pig oocytes [340]. Dehydroepiandrosterone activates energy metabolism in the theca and granulosa cells, thereby transferring energy to oocytes and promoting oocyte regeneration [341]. Growth hormone has a repairing effect on mitochondrial function in oocytes and can directly or indirectly promote oxidative stress balance and cellular antioxidant defense, as well as facilitate oxidative phosphorylation pathways [342]. FoxO3a is an important factor in regulating oocyte metabolism, and curcumin can regulate the PTEN/AKT/FoxO3a pathway to protect ovarian reserve [198]. However, further clinical and basic experiments are needed to determine the relevant mechanisms and clinical effects of these therapies.
Conclusions
The decline in fertility associated with women’s increasing age has become a significant issue for modern women [1,2,3,4]. As age increases, there is a decrease in ATP within the oocytes, leading to a decline in energy production capacity and mitochondrial function [8,9,10,11,12,13,14,15]. The changes in energy metabolism of oocytes with age have an impact on oocyte quality and are an important mechanism of reproductive aging [16,17,18]. However, it is still unclear how the changes in the intermediate steps of energy metabolism in oocytes affect ATP generation and how the related products and proteins influence oocyte quality, thus affecting ovarian aging. Therefore, this review summarizes the characteristics of oocyte energy metabolism, the changes in the TCA cycle, oxidative phosphorylation, lipid metabolism, glutamine metabolism, and the Adenosine remedial pathway in oocytes during age-related ovarian aging, as well as how these changes affect oocyte quality. This review also introduces the important proteins SIRTs and FoxO3a that regulate oocyte metabolism. Finally, this review discusses some treatment strategies for delaying ovarian aging, which may partially act by influencing oocyte energy metabolism. In conclusion, understanding the changes in oocyte metabolism and their influence on oocyte quality in age-related ovarian aging helps us comprehend the relationship between oocyte quality decline and the subsequent decline in fertility, and aids in identifying biomarkers and treatment methods.
Availability of data and materials
The datasets used and/or analyzed during the current study are available in the MEDLINE repository: https://pubmed.ncbi.nlm.nih.gov/.
All figures were depicted by PowerPoint.
Abbreviations
- ACAA:
-
3-Ketoacyl-CoA thiolase
- Acytl-CoA:
-
Acetyl coenzyme A
- ADP:
-
Adenosine diphosphate
- AKT:
-
Ak Strain Transforming
- AMP:
-
Adenosine monophosphate
- AMPK:
-
AMP-dependent protein kinase
- ANT1:
-
ADP/ATP translocase 1
- ATP:
-
Adenosine 5'-triphosphate
- CACT:
-
Carnitine–acylcarnitine–translocase
- CD36:
-
Cluster of Differentiation 36
- COX4:
-
Cytochrome c oxidase subunit 4
- CpG:
-
Cytosine-phosphate-guanine
- CPT1:
-
Carnitine Palmitoyltransferase 1
- CPT2:
-
Carnitine Palmitoyltransferase 1
- COX:
-
Cytochrome c oxidase subunit II
- CoQ:
-
Coenzyme Q
- DAG:
-
Diacylglycerol
- DGAT1:
-
Diacylglycerol o-acyltransferase 1
- DNA:
-
Deoxyribonucleic acid
- DNMT:
-
DNA methyltransferases
- DOR:
-
Diminished ovarian reserve
- EHHADH:
-
Enoyl-CoA hydratase 3-hydroxy acyl-CoA dehydrogenase
- eIF5A:
-
Eukaryotic translation initiation factor 5A
- ETC:
-
Electron transport chain
- FA:
-
Fatty acid
- FABP3:
-
Fatty acid-binding protein 3
- FADH2:
-
Flavin adenine dinucleotide
- FAO:
-
Fatty acid β-oxidation
- FATP:
-
Fatty acid transport protein
- FA-CoA:
-
Fatty acyl-CoA
- FOXO3a:
-
Forkhead box O3a
- GDH:
-
Glutamate dehydrogenase
- GH:
-
Growth hormone
- GLS:
-
Glutaminase
- GS:
-
Glutamine synthetase
- HDAC:
-
Histone Deacetylase
- HIF-1α:
-
Hypoxia-inducible factor-1α
- HOXD8:
-
Homeobox D8
- IGF:
-
Insulin-like Growth Factor
- KAT8:
-
Lysine acetyltransferase 8
- LCFA-CoA:
-
Long-chain acyl-CoAs
- LysoPL:
-
Lysophospholipid
- MAPKAP1:
-
Mitogen-activated protein Kinase Associated Protein 1
- MAPK14:
-
Mitogen-activated protein kinase 14
- MGE:
-
Mitochondrial genome editing
- MRPL:
-
Mitochondrial ribosomal protein L
- Mito:
-
Mitochondria
- mtDNA:
-
Mitochondrial DNA
- mTOR:
-
Mammalian Target of rapamycin
- NADH:
-
Nicotinamide adenine dinucleotide
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- ncRNA:
-
Non-coding RNA
- NMN:
-
β-Nicotinamide mononucleotide
- NR:
-
Nicotinamide riboside
- NR1D1:
-
Nuclear receptor subfamily 1, group D, member 1
- OXPHOS:
-
Oxidative phosphorylation
- PARP:
-
Poly(ADP-ribose) polymerase
- PDE:
-
Phosphodiesterase
- PI3K:
-
Phosphoinositide 3-kinase
- PL:
-
Phospholipid
- PMF:
-
Proton-motive force
- PPP:
-
Pentose phosphate pathway
- PPARA:
-
Peroxisome proliferator-activated receptor alpha
- rDNA:
-
Ribosomal DNA
- ROS:
-
Reactive oxygen species
- SAT1:
-
Spermidine/spermine N1-acetyltransferase
- SIRTs:
-
Sirtuin enzyme family
- SLC14A1:
-
Solute carrier family 14 member 1
- TAGs:
-
Triacylglycerols
- TCA cycle:
-
Tricarboxylic acid cycle
- Tcf20:
-
Transcription co-activator factor 20
- 5-mC:
-
5-Methylcytosine
References
te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–54.
Morabia A, Costanza MC. International variability in ages at menarche, first livebirth, and menopause World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives. Am J Epidemiol. 1998;148(12):1195–205.
Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–60.
Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233(4771):1389–94.
Mottis A, Herzig S, Auwerx J. Mitocellular communication: Sha** health and disease. Science. 2019;366(6467):827–32.
Merry TL, Chan A, Woodhead JST, Reynolds JC, Kumagai H, Kim SJ, et al. Mitochondrial-derived peptides in energy metabolism. Am J Physiol Endocrinol Metab. 2020;319(4):E659–66.
Smits MAJ, Schomakers BV, van Weeghel M, Wever EJM, Wüst RCI, Dijk F, et al. Human ovarian aging is characterized by oxidative damage and mitochondrial dysfunction. Hum Reprod. 2023;38:2208–20.
Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, et al. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev. 2011;23(3):424–32.
Simsek-Duran F, Li F, Ford W, Swanson RJ, Jones HW Jr, Castora FJ. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS ONE. 2013;8(5):e64955.
Rigoulet M, Bouchez CL, Paumard P, Ransac S, Cuvellier S, Duvezin-Caubet S, et al. Cell energy metabolism: An update. Biochim Biophys Acta Bioenerg. 2020;1861(11):148276.
Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14(5):887–95.
Catandi GD, Obeidat YM, Broeckling CD, Chen TW, Chicco AJ, Carnevale EM. Equine maternal aging affects oocyte lipid content, metabolic function and developmental potential. Reproduction. 2021;161(4):399–409.
Catandi GD, Bresnahan DR, Peters SO, Fresa KJ, Maclellan LJ, Broeckling CD, et al. Equine maternal aging affects the metabolomic profile of oocytes and follicular cells during different maturation time points. Front Cell Dev Biol. 2023;11:1239154.
Rambags BP, van Boxtel DC, Tharasanit T, Lenstra JA, Colenbrander B, Stout TA. Advancing maternal age predisposes to mitochondrial damage and loss during maturation of equine oocytes in vitro. Theriogenology. 2014;81(7):959–65.
Huang J, Chen P, Jia L, Li T, Yang X, Liang Q, et al. Multi-Omics Analysis Reveals Translational Landscapes and Regulations in Mouse and Human Oocyte Aging. Adv Sci Weinh. 2023;10(26):e2301538.
Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10(2):415–24.
Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813.
Eichenlaub-Ritter U. Oocyte ageing and its cellular basis. Int J Dev Biol. 2012;56(10–12):841–52.
Borkum JM. The Tricarboxylic Acid Cycle as a Central Regulator of the Rate of Aging: Implications for Metabolic Interventions. Adv Biol (Weinh). 2023;7(7):e2300095.
Sharma R, Ramanathan A. The Aging Metabolome-Biomarkers to Hub Metabolites. Proteomics. 2020;20(5–6):e1800407.
Herta AC, von Mengden L, Akin N, Billooye K, Coucke W, van Leersum J, et al. Characterization of carbohydrate metabolism in in vivo- and in vitro-grown and matured mouse antral follicles†. Biol Reprod. 2022;107(4):998–1013.
Zhang H, Li C, Wen D, Li R, Lu S, Xu R, et al. Melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply. Redox Biol. 2022;49:102215.
Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, et al. Single-Cell Transcriptomic Atlas of Primate Ovarian Aging. Cell. 2020;180(3):585–600.e19.
Schultz MB, Sinclair DA. Why NAD(+) Declines during Aging: It’s Destroyed. Cell Metab. 2016;23(6):965–6.
Johnson S, Imai SI. NAD (+) biosynthesis, aging, and disease. F1000Res. 2018;7:132.
Smits MAJ, Schomakers BV, van Weeghel M, Wever EJM, Wüst RCI, Dijk F, et al. Human ovarian aging is characterized by oxidative damage and mitochondrial dysfunction. Hum Reprod. 2023;38(11):2208–20.
Rajman L, Chwalek K, Sinclair DA. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018;27(3):529–47.
Clement J, Wong M, Poljak A, Sachdev P, Braidy N. The Plasma NAD(+) Metabolome Is Dysregulated in “Normal” Aging. Rejuvenation Res. 2019;22(2):121–30.
Baccolo G, Stamerra G, Coppola DP, Orlandi I, Vai M. Mitochondrial Metabolism and Aging in Yeast. Int Rev Cell Mol Biol. 2018;340:1–33.
Huang Q, Sun M, Li M, Zhang D, Han F, Wu JC, et al. Combination of NAD(+) and NADPH Offers Greater Neuroprotection in Ischemic Stroke Models by Relieving Metabolic Stress. Mol Neurobiol. 2018;55(7):6063–75.
Poljšak B, Kovač V, Špalj S, Milisav I. The Central Role of the NAD+ Molecule in the Development of Aging and the Prevention of Chronic Age-Related Diseases: Strategies for NAD+ Modulation. Int J Mol Sci. 2023;24(3):2959.
Yang Q, Li H, Wang H, Chen W, Zeng X, Luo X, et al. Deletion of enzymes for de novo NAD(+) biosynthesis accelerated ovarian aging. Aging Cell. 2023;22(9):e13904.
Yang Q, Cong L, Wang Y, Luo X, Li H, Wang H, et al. Increasing ovarian NAD(+) levels improve mitochondrial functions and reverse ovarian aging. Free Radic Biol Med. 2020;156:1–10.
Salminen A, Kaarniranta K, Hiltunen M, Kauppinen A. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: novel insights into mitochondrial regulation of aging process. Cell Signal. 2014;26(7):1598–603.
Salminen A, Kauppinen A, Kaarniranta K. 2-Oxoglutarate-dependent dioxygenases are sensors of energy metabolism, oxygen availability, and iron homeostasis: potential role in the regulation of aging process. Cell Mol Life Sci. 2015;72(20):3897–914.
Akino N, Wada-Hiraike O, Isono W, Terao H, Honjo H, Miyamoto Y, et al. Activation of Nrf2/Keap1 pathway by oral Dimethylfumarate administration alleviates oxidative stress and age-associated infertility might be delayed in the mouse ovary. Reprod Biol Endocrinol. 2019;17(1):23.
He H, Wang J, Mou X, Liu X, Li Q, Zhong M, et al. Selective autophagic degradation of ACLY (ATP citrate lyase) maintains citrate homeostasis and promotes oocyte maturation. Autophagy. 2023;19(1):163–79.
Lee HC, Wei YH. Mitochondria and aging. Adv Exp Med Biol. 2012;942:311–27.
Cinco R, Digman MA, Gratton E, Luderer U. Spatial Characterization of Bioenergetics and Metabolism of Primordial to Preovulatory Follicles in Whole Ex Vivo Murine Ovary. Biol Reprod. 2016;95(6):129.
Zhang T, ** Q, Wang D, Li J, Wang M, Li D, et al. Mitochondrial dysfunction and endoplasmic reticulum stress involved in oocyte aging: an analysis using single-cell RNA-sequencing of mouse oocytes. J Ovarian Res. 2019;12(1):53.
Vercellino I, Sazanov LA. The assembly, regulation and function of the mitochondrial respiratory chain. Nat Rev Mol Cell Biol. 2022;23(2):141–61.
Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64(3):577–83.
Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20(6):591–8.
Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Döring F. Functions of coenzyme Q10 in inflammation and gene expression. BioFactors. 2008;32(1–4):179–83.
Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuñiga O, Delgado-Lista J, Gutierrez-Mariscal FM, Perez-Martinez P, et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol A Biol Sci Med Sci. 2012;67(1):3–10.
Li X, Zhan J, Hou Y, Hou Y, Chen S, Luo D, et al. Coenzyme Q10 Regulation of Apoptosis and Oxidative Stress in H(2)O(2) Induced BMSC Death by Modulating the Nrf-2/NQO-1 Signaling Pathway and Its Application in a Model of Spinal Cord Injury. Oxid Med Cell Longev. 2019;2019:6493081.
Rodríguez-Varela C, Labarta E. Does Coenzyme Q10 Supplementation Improve Human Oocyte Quality? Int J Mol Sci. 2021;22(17):9541.
Mailloux RJ. An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants (Basel). 2020;9(6):472.
Chenna S, Koopman WJH, Prehn JHM, Connolly NMC. Mechanisms and mathematical modeling of ROS production by the mitochondrial electron transport chain. Am J Physiol Cell Physiol. 2022;323(1):C69–c83.
Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–5.
Kadenbach B, Complex IV. The regulatory center of mitochondrial oxidative phosphorylation. Mitochondrion. 2021;58:296–302.
Ayer A, Fazakerley DJ, Suarna C, Maghzal GJ, Sheipouri D, Lee KJ, et al. Genetic screening reveals phospholipid metabolism as a key regulator of the biosynthesis of the redox-active lipid coenzyme Q. Redox Biol. 2021;46:102127.
Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. 2016;23:36.
Wang L, Tang J, Wang L, Tan F, Song H, Zhou J, et al. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol. 2021;236(12):7966–83.
Li Q, Mu L, Yang X, Wang G, Liang J, Wang S, et al. Discovery of Oogenesis Biomarkers from Mouse Oocytes Using a Single-Cell Proteomics Approach. J Proteome Res. 2023;22(6):2067–78.
Jiao X, Liu N, Xu Y, Qiao H. Perfluorononanoic acid impedes mouse oocyte maturation by inducing mitochondrial dysfunction and oxidative stress. Reprod Toxicol. 2021;104:58–67.
Tsang WY, Sayles LC, Grad LI, Pilgrim DB, Lemire BD. Mitochondrial respiratory chain deficiency in Caenorhabditis elegans results in developmental arrest and increased life span. J Biol Chem. 2001;276(34):32240–6.
Curran SP, Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3(4):e56.
Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 2018;19(2):109–20.
Trushina E, Trushin S, Hasan MF. Mitochondrial complex I as a therapeutic target for Alzheimer’s disease. Acta Pharm Sin B. 2022;12(2):483–95.
Seli E, Wang T, Horvath TL. Mitochondrial unfolded protein response: a stress response with implications for fertility and reproductive aging. Fertil Steril. 2019;111(2):197–204.
Qi X, Rusch NJ, Fan J, Mora CJ, **e L, Mu S, et al. Mitochondrial proton leak in cardiac aging. Geroscience. 2023;45:2135–43.
Zhang H, Alder NN, Wang W, Szeto H, Marcinek DJ, Rabinovitch PS. Reduction of elevated proton leak rejuvenates mitochondria in the aged cardiomyocyte. Elife. 2020;9:e60827.
Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510(7505):397–401.
Zhang W, Wu F. Effects of adverse fertility-related factors on mitochondrial DNA in the oocyte: a comprehensive review. Reprod Biol Endocrinol. 2023;21(1):27.
Nie J, Yan K, Sui L, Zhang H, Zhang H, Yang X, et al. Mogroside V improves porcine oocyte in vitro maturation and subsequent embryonic development. Theriogenology. 2020;141:35–40.
Cagnone GL, Tsai TS, Makanji Y, Matthews P, Gould J, Bonkowski MS, et al. Restoration of normal embryogenesis by mitochondrial supplementation in pig oocytes exhibiting mitochondrial DNA deficiency. Sci Rep. 2016;6:23229.
Cozzolino M, Marin D, Sisti G. New Frontiers in IVF: mtDNA and autologous germline mitochondrial energy transfer. Reprod Biol Endocrinol. 2019;17(1):55.
Lewis N, Hinrichs K, Leese HJ, Mc GAC, Brison DR, Sturmey R. Energy metabolism of the equine cumulus oocyte complex during in vitro maturation. Sci Rep. 2020;10(1):3493.
Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, Robker RL. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod. 2010;83(6):909–18.
Montjean D, Entezami F, Lichtblau I, Belloc S, Gurgan T, Menezo Y. Carnitine content in the follicular fluid and expression of the enzymes involved in beta oxidation in oocytes and cumulus cells. J Assist Reprod Genet. 2012;29(11):1221–5.
Cetica P, Pintos L, Dalvit G, Beconi M. Involvement of enzymes of amino acid metabolism and tricarboxylic acid cycle in bovine oocyte maturation in vitro. Reproduction. 2003;126(6):753–63.
Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation. Reproduction. 2014;148(1):R15–27.
Um DE, Shin H, Park D, Ahn JM, Kim J, Song H, et al. Molecular analysis of lipid uptake- and necroptosis-associated factor expression in vitrified-warmed mouse oocytes. Reprod Biol Endocrinol. 2020;18(1):37.
Yazigi RA, Chi MM, Mastrogiannis DS, Strickler RC, Yang VC, Lowry OH. Enzyme activities and maturation in unstimulated and exogenous gonadotropin-stimulated human oocytes. Am J Physiol. 1993;264(4 Pt 1):C951–5.
Cañón-Beltrán K, Giraldo-Giraldo J, Cajas YN, Beltrán-Breña P, Hidalgo CO, Vásquez N, et al. Inhibiting diacylglycerol acyltransferase-1 reduces lipid biosynthesis in bovine blastocysts produced in vitro. Theriogenology. 2020;158:267–76.
Su GM, Fiala-Beer E, Weber J, Jahn D, Robertson GR, Murray M. Pretranslational upregulation of microsomal CYP4A in rat liver by intake of a high-sucrose, lipid-devoid diet containing orotic acid. Biochem Pharmacol. 2005;69(4):709–17.
Koo SH. Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol. 2013;19(3):210–5.
Li JX, Ke DZ, Yao L, Wang S, Ma P, Liu L, et al. Response of genes involved in lipid metabolism in rat epididymal white adipose tissue to different fasting conditions after long-term fructose consumption. Biochem Biophys Res Commun. 2017;484(2):336–41.
Bougarne N, Weyers B, Desmet SJ, Deckers J, Ray DW, Staels B, et al. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr Rev. 2018;39(5):760–802.
Zhao L, Zhang C, Luo X, Wang P, Zhou W, Zhong S, et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J Hepatol. 2018;69(3):705–17.
Glatz JFC, Heather LC, Luiken J. CD36 as a gatekeeper of myocardial lipid metabolism and therapeutic target for metabolic disease. Physiol Rev. 2024;104(2):727–64.
Li Y, Huang X, Yang G, Xu K, Yin Y, Brecchia G, et al. CD36 favours fat sensing and transport to govern lipid metabolism. Prog Lipid Res. 2022;88:101193.
Del Collado M, da Silveira JC, Sangalli JR, Andrade GM, Sousa L, Silva LA, et al. Fatty Acid Binding Protein 3 And Transzonal Projections Are Involved In Lipid Accumulation During In Vitro Maturation Of Bovine Oocytes. Sci Rep. 2017;7(1):2645.
Paczkowski M, Schoolcraft WB, Krisher RL. Fatty acid metabolism during maturation affects glucose uptake and is essential to oocyte competence. Reproduction. 2014;148(4):429–39.
Agarwal A, Sengupta P, Durairajanayagam D. Role of L-carnitine in female infertility. Reprod Biol Endocrinol. 2018;16(1):5.
Placidi M, Vergara T, Casoli G, Flati I, Capece D, Artini PG, et al. Acyl-Carnitines Exert Positive Effects on Mitochondrial Activity under Oxidative Stress in Mouse Oocytes: A Potential Mechanism Underlying Carnitine Efficacy on PCOS. Biomedicines. 2023;11(9):2474.
Berteli TS, Vireque AA, Borges ED, Da Luz CM, Navarro PA. Membrane lipid changes in mouse blastocysts induced by ovarian stimulation, IVF and oocyte vitrification. Reprod Biomed Online. 2023;46(6):887–902.
Placidi M, Di Emidio G, Virmani A, D’Alfonso A, Artini PG, D’Alessandro AM, et al. Carnitines as Mitochondrial Modulators of Oocyte and Embryo Bioenergetics. Antioxidants (Basel). 2022;11(4):745.
Paczkowski M, Silva E, Schoolcraft WB, Krisher RL. Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biol Reprod. 2013;88(5):111.
Valsangkar D, Downs SM. A requirement for fatty acid oxidation in the hormone-induced meiotic maturation of mouse oocytes. Biol Reprod. 2013;89(2):43.
Pawlak P, Lipinska P, Sell-Kubiak E, Kajdasz A, Derebecka N, Warzych E. Energy metabolism disorders during in vitro maturation of bovine cumulus-oocyte complexes interfere with blastocyst quality and metabolism. Dev Biol. 2024;509:51–8.
Chung KW. Advances in Understanding of the Role of Lipid Metabolism in Aging. Cells. 2021;10(4):880.
Johnson AA, Stolzing A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell. 2019;18(6):e13048.
Baccouch R, Shi Y, Vernay E, Mathelié-Guinlet M, Taib-Maamar N, Villette S, et al. The impact of lipid polyunsaturation on the physical and mechanical properties of lipid membranes. Biochim Biophys Acta Biomembr. 2023;1865(2):184084.
Wang Y, Pope I, Brennan-Craddock H, Poole E, Langbein W, Borri P, et al. A primary effect of palmitic acid on mouse oocytes is the disruption of the structure of the endoplasmic reticulum. Reproduction. 2021;163(1):45–56.
Diskin C, Ryan TAJ, O’Neill LAJ. Modification of Proteins by Metabolites in Immunity. Immunity. 2021;54(1):19–31.
Nolan CJ, Larter CZ. Lipotoxicity: why do saturated fatty acids cause and monounsaturates protect against it? J Gastroenterol Hepatol. 2009;24(5):703–6.
Valckx SD, Van Hoeck V, Arias-Alvarez M, Maillo V, Lopez-Cardona AP, Gutierrez-Adan A, et al. Elevated non-esterified fatty acid concentrations during in vitro murine follicle growth alter follicular physiology and reduce oocyte developmental competence. Fertil Steril. 2014;102(6):1769–76.e1.
Stoffel W, Schmidt-Soltau I, Binczek E, Thomas A, Thevis M, Wegner I. Dietary ω3-and ω6-Polyunsaturated fatty acids reconstitute fertility of Juvenile and adult Fads2-Deficient mice. Mol Metab. 2020;36:100974.
Li Y, Li X, Ye D, Zhang R, Liu C, He M, et al. Endogenous biosynthesis of docosahexaenoic acid (DHA) regulates fish oocyte maturation by promoting pregnenolone production. Zool Res. 2024;45(1):176–88.
Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell. 2012;11(6):1046–54.
Ma R, Wang S, Xue M, Zhang H, He Z, Jueraitetibaike K, et al. Effects of n-3 PUFA supplementation on oocyte in vitro maturation in mice with polycystic ovary syndrome. J Ovarian Res. 2023;16(1):87.
Freret S, Oseikria M, Bourhis DL, Desmarchais A, Briant E, Desnoes O, et al. Effects of a n-3 polyunsaturated fatty acid-enriched diet on embryo production in dairy cows. Reproduction. 2019;158(1):71–83.
Chiu YH, Karmon AE, Gaskins AJ, Arvizu M, Williams PL, Souter I, et al. Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum Reprod. 2018;33(1):156–65.
Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am J Physiol Endocrinol Metab. 2008;294(2):E425–34.
Ruiz-Sanz JI, Pérez-Ruiz I, Meijide S, Ferrando M, Larreategui Z, Ruiz-Larrea MB. Lower follicular n-3 polyunsaturated fatty acid levels are associated with a better response to ovarian stimulation. J Assist Reprod Genet. 2019;36(3):473–82.
Oseikria M, Elis S, Maillard V, Corbin E, Uzbekova S. N-3 polyunsaturated fatty acid DHA during IVM affected oocyte developmental competence in cattle. Theriogenology. 2016;85(9):1625–34.e2.
Pawlak P, Malyszka N, Szczerbal I, Kolodziejski P. Fatty acid induced lipolysis influences embryo development, gene expression and lipid droplet formation in the porcine cumulus cells†. Biol Reprod. 2020;103(1):36–48.
Ciepiela P, Bączkowski T, Drozd A, Kazienko A, Stachowska E, Kurzawa R. Arachidonic and linoleic acid derivatives impact oocyte ICSI fertilization–a prospective analysis of follicular fluid and a matched oocyte in a “one follicle–one retrieved oocyte–one resulting embryo” investigational setting. PLoS ONE. 2015;10(3):e0119087.
Marei WF, Wathes DC, Fouladi-Nashta AA. Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction. 2010;139(6):979–88.
Ghaffarilaleh V, Fouladi-Nashta A, Paramio MT. Effect of α-linolenic acid on oocyte maturation and embryo development of prepubertal sheep oocytes. Theriogenology. 2014;82(5):686–96.
Dang L, Dong Y, Zhang C, Su B, Ning N, Zhou S, et al. Zishen Yutai pills restore fertility in premature ovarian failure through regulating arachidonic acid metabolism and the ATK pathway. J Ethnopharmacol. 2024;324:117782.
Prates EG, Nunes JT, Pereira RM. A role of lipid metabolism during cumulus-oocyte complex maturation: impact of lipid modulators to improve embryo production. Mediators Inflamm. 2014;2014:692067.
Van Hoeck V, Rizos D, Gutierrez-Adan A, Pintelon I, Jorssen E, Dufort I, et al. Interaction between differential gene expression profile and phenotype in bovine blastocysts originating from oocytes exposed to elevated non-esterified fatty acid concentrations. Reprod Fertil Dev. 2015;27(2):372–84.
Shi M, Sirard MA. Transcriptome and epigenome analysis of porcine embryos from non-esterified fatty acid-exposed oocytes. Domest Anim Endocrinol. 2021;76:106605.
Shi M, Sirard MA. Cocultured porcine granulosa cells respond to excess non-esterified fatty acids during in vitro maturation. J Ovarian Res. 2021;14(1):142.
Yang X, Wu LL, Chura LR, Liang X, Lane M, Norman RJ, et al. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil Steril. 2012;97(6):1438–43.
Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab. 2009;94(5):1533–40.
Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol. 2011;88(2):142–8.
Liu Y, Tilleman K, Vlaeminck B, Gervais R, Chouinard PY, De Sutter P, et al. The fatty acid composition in follicles is related to the developmental potential of oocytes up to the blastocyst stage: a single-centre cohort study. Reprod Biol Endocrinol. 2022;20(1):107.
Shi M, Sirard MA. Metabolism of fatty acids in follicular cells, oocytes, and blastocysts. Reprod Fertil. 2022;3(2):R96–r108.
Van Hoeck V, Sturmey RG, Bermejo-Alvarez P, Rizos D, Gutierrez-Adan A, Leese HJ, et al. Elevated non-esterified fatty acid concentrations during bovine oocyte maturation compromise early embryo physiology. PLoS ONE. 2011;6(8):e23183.
Desmet KL, Van Hoeck V, Gagné D, Fournier E, Thakur A, O’Doherty AM, et al. Exposure of bovine oocytes and embryos to elevated non-esterified fatty acid concentrations: integration of epigenetic and transcriptomic signatures in resultant blastocysts. BMC Genomics. 2016;17(1):1004.
Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE. 2010;5(4):e10074.
Aardema H, Lolicato F, van de Lest CH, Brouwers JF, Vaandrager AB, van Tol HT, et al. Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intracellular lipid storage. Biol Reprod. 2013;88(6):164.
Zhang J, Pavlova NN, Thompson CB. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. Embo j. 2017;36(10):1302–15.
Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Exp Mol Med. 2020;52(9):1496–516.
Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56(3):414–24.
Cruzat V, MacedoRogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018;10(11):1564.
Steeves TE, Gardner DK. Metabolism of glucose, pyruvate, and glutamine during the maturation of oocytes derived from pre-pubertal and adult cows. Mol Reprod Dev. 1999;54(1):92–101.
**ao D, Zeng L, Yao K, Kong X, Wu G, Yin Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids. 2016;48(9):2067–80.
Johmura Y, Yamanaka T, Omori S, Wang TW, Sugiura Y, Matsumoto M, et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science. 2021;371(6526):265–70.
Choudhury D, Rong N, Ikhapoh I, Rajabian N, Tseropoulos G, Wu Y, et al. Inhibition of glutaminolysis restores mitochondrial function in senescent stem cells. Cell Rep. 2022;41(9):111744.
Lian G, Gnanaprakasam JR, Wang T, Wu R, Chen X, Liu L, et al. Glutathione de novo synthesis but not recycling process coordinates with glutamine catabolism to control redox homeostasis and directs murine T cell differentiation. Elife. 2018;7:e36158.
Garnham CP, Roll-Mecak A. The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton (Hoboken). 2012;69(7):442–63.
Lamas-Toranzo I, Pericuesta E, Bermejo-Álvarez P. Mitochondrial and metabolic adjustments during the final phase of follicular development prior to IVM of bovine oocytes. Theriogenology. 2018;119:156–62.
Kang T, Zhao S, Shi L, Li J. Glucose metabolism is required for oocyte maturation of zebrafish. Biochem Biophys Res Commun. 2021;559:191–6.
**e HL, Wang YB, Jiao GZ, Kong DL, Li Q, Li H, et al. Effects of glucose metabolism during in vitro maturation on cytoplasmic maturation of mouse oocytes. Sci Rep. 2016;6:20764.
Li Q, Miao DQ, Zhou P, Wu YG, Gao D, Wei DL, et al. Glucose metabolism in mouse cumulus cells prevents oocyte aging by maintaining both energy supply and the intracellular redox potential. Biol Reprod. 2011;84(6):1111–8.
Akin N, von Mengden L, Herta AC, Billooye K, van Leersum J, Cava-Cami B, et al. Glucose metabolism characterization during mouse in vitro maturation identifies alterations in cumulus cells†. Biol Reprod. 2021;104(4):902–13.
Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139(4):685–95.
Kuchiiwa T, Nio-Kobayashi J, Takahashi-Iwanaga H, Yajima T, Iwanaga T. Cellular expression of monocarboxylate transporters in the female reproductive organ of mice: implications for the genital lactate shuttle. Histochem Cell Biol. 2011;135(4):351–60.
**e HL, Zhu S, Zhang J, Wen J, Yuan HJ, Pan LZ, et al. Glucose metabolism during in vitro maturation of mouse oocytes: An study using RNA interference. J Cell Physiol. 2018;233(9):6952–64.
Fontana J, Martínková S, Petr J, Žalmanová T, Trnka J. Metabolic cooperation in the ovarian follicle. Physiol Res. 2020;69(1):33–48.
Richani D, Dunning KR, Thompson JG, Gilchrist RB. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update. 2021;27(1):27–47.
Kansaku K, Itami N, Kawahara-Miki R, Shirasuna K, Kuwayama T, Iwata H. Differential effects of mitochondrial inhibitors on porcine granulosa cells and oocytes. Theriogenology. 2017;103:98–103.
Alvarez GM, Casiró S, Gutnisky C, Dalvit GC, Sutton-McDowall ML, Thompson JG, et al. Implications of glycolytic and pentose phosphate pathways on the oxidative status and active mitochondria of the porcine oocyte during IVM. Theriogenology. 2016;86(9):2096–106.
Liu N, Wu YG, Lan GC, Sui HS, Ge L, Wang JZ, et al. Pyruvate prevents aging of mouse oocytes. Reproduction. 2009;138(2):223–34.
Funahashi H, Koike T, Sakai R. Effect of glucose and pyruvate on nuclear and cytoplasmic maturation of porcine oocytes in a chemically defined medium. Theriogenology. 2008;70(7):1041–7.
Gutnisky C, Dalvit GC, Thompson JG, Cetica PD. Pentose phosphate pathway activity: effect on in vitro maturation and oxidative status of bovine oocytes. Reprod Fertil Dev. 2014;26(7):931–42.
TeSlaa T, Ralser M, Fan J, Rabinowitz JD. The pentose phosphate pathway in health and disease. Nat Metab. 2023;5(8):1275–89.
Hou X, Zhang L, Han L, Ge J, Ma R, Zhang X, et al. Differing roles of pyruvate dehydrogenase kinases during mouse oocyte maturation. J Cell Sci. 2015;128(13):2319–29.
Purcell SH, Chi MM, Lanzendorf S, Moley KH. Insulin-stimulated glucose uptake occurs in specialized cells within the cumulus oocyte complex. Endocrinology. 2012;153(5):2444–54.
Li Q, Wang G, Zhang J, Zhou P, Wang TY, Cui W, et al. Combined inhibitory effects of pyruvate and low temperature on postovulatory aging of mouse oocytes. Biol Reprod. 2012;87(5):105.
da AlcantaraSilva JV, Ispada J, Nociti RP, da Fonseca Junior AM, de Lima CB, Dos Santos EC, et al. The central role of pyruvate metabolism on the epigenetic maturation and transcriptional profile of bovine oocytes. Reproduction. 2024;167(4):e230181.
Campbell JM, Mahbub SB, Bertoldo MJ, Habibalahi A, Goss DM, Ledger WL, et al. Multispectral autofluorescence characteristics of reproductive aging in old and young mouse oocytes. Biogerontology. 2022;23(2):237–49.
Luo LL, Chen XC, Fu YC, Xu JJ, Li L, Lin XH, et al. The effects of caloric restriction and a high-fat diet on ovarian lifespan and the expression of SIRT1 and SIRT6 proteins in rats. Aging Clin Exp Res. 2012;24(2):125–33.
Di Emidio G, Falone S, Vitti M, D’Alessandro AM, Vento M, Di Pietro C, et al. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod. 2014;29(9):2006–17.
Zhang T, Zhou Y, Li L, Wang HH, Ma XS, Qian WP, et al. SIRT1, 2, 3 protect mouse oocytes from postovulatory aging. Aging (Albany NY). 2016;8(4):685–96.
Qiu D, Hou X, Han L, Li X, Ge J, Wang Q. Sirt2-BubR1 acetylation pathway mediates the effects of advanced maternal age on oocyte quality. Aging Cell. 2018;17(1):e12698.
Zhu J, Yang Q, Li H, Wang Y, Jiang Y, Wang H, et al. Sirt3 deficiency accelerates ovarian senescence without affecting spermatogenesis in aging mice. Free Radic Biol Med. 2022;193(Pt 2):511–25.
Jiang WJ, Yao XR, Zhao YH, Gao QS, ** QG, Li YH, et al. L-carnitine prevents bovine oocyte aging and promotes subsequent embryonic development. J Reprod Dev. 2019;65(6):499–506.
Manosalva I, González A. Aging changes the chromatin configuration and histone methylation of mouse oocytes at germinal vesicle stage. Theriogenology. 2010;74(9):1539–47.
Zeng J, Jiang M, Wu X, Diao F, Qiu D, Hou X, et al. SIRT4 is essential for metabolic control and meiotic structure during mouse oocyte maturation. Aging Cell. 2018;17(4):e12789.
Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105(38):14447–52.
Rahman M, Nirala NK, Singh A, Zhu LJ, Taguchi K, Bamba T, et al. Drosophila Sirt2/mammalian SIRT3 deacetylates ATP synthase β and regulates complex V activity. J Cell Biol. 2014;206(2):289–305.
Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49(2):304–11.
Chen DD, Shi Q, Liu X, Liang DL, Wu YZ, Fan Q, et al. Aberrant SENP1-SUMO-Sirt3 signaling causes the disturbances of mitochondrial deacetylation and oxidative phosphorylation in prion-infected animal and cell models. ACS Chem Neurosci. 2023;14(9):1610–21.
Sun Y, Tian Z, Liu N, Zhang L, Gao Z, Sun X, et al. Exogenous H(2)S switches cardiac energy substrate metabolism by regulating SIRT3 expression in db/db mice. J Mol Med (Berl). 2018;96(3–4):281–99.
**g E, O’Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes. 2013;62(10):3404–17.
Xu Y, Zhang S, Rong J, Lin Y, Du L, Wang Y, et al. Sirt3 is a novel target to treat sepsis induced myocardial dysfunction by acetylated modulation of critical enzymes within cardiac tricarboxylic acid cycle. Pharmacol Res. 2020;159:104887.
Di Emidio G, Falone S, Artini PG, Amicarelli F, D’Alessandro AM, Tatone C. Mitochondrial Sirtuins in Reproduction. Antioxidants (Basel). 2021;10(7):1047.
Ho L, Titus AS, Banerjee KK, George S, Lin W, Deota S, et al. SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK. Aging (Albany NY). 2013;5(11):835–49.
Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126(5):941–54.
Mathias RA, Greco TM, Oberstein A, Budayeva HG, Chakrabarti R, Rowland EA, et al. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell. 2014;159(7):1615–25.
Laurent G, German NJ, Saha AK, de Boer VC, Davies M, Koves TR, et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell. 2013;50(5):686–98.
Guo L, Zhou SR, Wei XB, Liu Y, Chang XX, Liu Y, et al. Acetylation of Mitochondrial Trifunctional Protein α-Subunit Enhances Its Stability To Promote Fatty Acid Oxidation and Is Decreased in Nonalcoholic Fatty Liver Disease. Mol Cell Biol. 2016;36(20):2553–67.
Smirnov D, Eremenko E, Stein D, Kaluski S, Jasinska W, Cosentino C, et al. SIRT6 is a key regulator of mitochondrial function in the brain. Cell Death Dis. 2023;14(1):35.
Li Y, Miao Y, Chen J, **ong B. SIRT6 Maintains Redox Homeostasis to Promote Porcine Oocyte Maturation. Front Cell Dev Biol. 2021;9:625540.
Xu D, He H, Liu D, Geng G, Li Q. A novel role of SIRT2 in regulating gap junction communications via connexin-43 in bovine cumulus-oocyte complexes. J Cell Physiol. 2020;235(10):7332–43.
Xu D, Wu L, Jiang X, Yang L, Cheng J, Chen H, et al. SIRT2 Inhibition Results in Meiotic Arrest, Mitochondrial Dysfunction, and Disturbance of Redox Homeostasis during Bovine Oocyte Maturation. Int J Mol Sci. 2019;20(6):1365.
Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32(5):1183–94.
Osum M, Serakinci N. Impact of circadian disruption on health; SIRT1 and Telomeres. DNA Repair (Amst). 2020;96:102993.
Di Emidio G, Santini SJ, D’Alessandro AM, Vetuschi A, Sferra R, Artini PG, et al. SIRT1 participates in the response to methylglyoxal-dependent glycative stress in mouse oocytes and ovary. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1389–401.
Grzeczka A, Kordowitzki P. Resveratrol and SIRT1: Antiaging Cornerstones for Oocytes? Nutrients. 2022;14(23):5101.
Luo C, Zhang J, Bo L, Wei L, Yang G, Gao S, et al. Construction of a ceRNA-based lncRNA-mRNA network to identify functional lncRNAs in premature ovarian insufficiency. Front Genet. 2022;13:956805.
Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274(23):15982–5.
Morris BJ, Willcox DC, Donlon TA, Willcox BJ. FOXO3: A Major Gene for Human Longevity–A Mini-Review. Gerontology. 2015;61(6):515–25.
Zhong S, Chen W, Wang B, Gao C, Liu X, Song Y, et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis. Redox Biol. 2023;63:102760.
Zhou K, Wei Y, Li X, Yang X. MiR-223-3p targets FOXO3a to inhibit radiosensitivity in prostate cancer by activating glycolysis. Life Sci. 2021;282:119798.
He Q, Yin J, Zou B, Guo H. WIN55212-2 alleviates acute lung injury by inhibiting macrophage glycolysis through the miR-29b-3p/FOXO3/PFKFB3 axis. Mol Immunol. 2022;149:119–28.
Luo B, Wu Y, Liu SL, Li XY, Zhu HR, Zhang L, et al. Vagus nerve stimulation optimized cardiomyocyte phenotype, sarcomere organization and energy metabolism in infarcted heart through FoxO3A-VEGF signaling. Cell Death Dis. 2020;11(11):971.
Dong Z, Yang J, Li L, Tan L, Shi P, Zhang J, et al. FOXO3a-SIRT6 axis suppresses aerobic glycolysis in melanoma. Int J Oncol. 2020;56(3):728–42.
Jacobs KM, Pennington JD, Bisht KS, Aykin-Burns N, Kim HS, Mishra M, et al. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int J Biol Sci. 2008;4(5):291–9.
Zhuan Q, Li J, Du X, Zhang L, Meng L, Cheng K, et al. Nampt affects mitochondrial function in aged oocytes by mediating the downstream effector FoxO3a. J Cell Physiol. 2022;237(1):647–59.
Zhang H, Lin F, Zhao J, Wang Z. Expression Regulation and Physiological Role of Transcription Factor FOXO3a During Ovarian Follicular Development. Front Physiol. 2020;11:595086.
Lv Y, Cao RC, Liu HB, Su XW, Lu G, Ma JL, et al. Single-Oocyte Gene Expression Suggests That Curcumin Can Protect the Ovarian Reserve by Regulating the PTEN-AKT-FOXO3a Pathway. Int J Mol Sci. 2021;22(12):6570.
Pelosi E, Omari S, Michel M, Ding J, Amano T, Forabosco A, et al. Constitutively active Foxo3 in oocytes preserves ovarian reserve in mice. Nat Commun. 2013;4:1843.
Wang M, Ren J, Chen X, Liu J, Xu X, Li X, et al. 20(S)-ginsenoside Rg3 promotes myoblast differentiation and protects against myotube atrophy via regulation of the Akt/mTOR/FoxO3 pathway. Biochem Pharmacol. 2020;180:114145.
Saccon TD, Moreira F, Cruz LA, Mondadori RG, Fang Y, Barros CC, et al. Ovarian aging and the activation of the primordial follicle reserve in the long-lived Ames dwarf and the short-lived bGH transgenic mice. Mol Cell Endocrinol. 2017;455:23–32.
Bhardwaj G, Penniman CM, Jena J, Suarez Beltran PA, Foster C, Poro K, et al. Insulin and IGF-1 receptors regulate complex I-dependent mitochondrial bioenergetics and supercomplexes via FoxOs in muscle. J Clin Invest. 2021;131(18):e146415.
Nebbioso A, Tambaro FP, Dell’Aversana C, Altucci L. Cancer epigenetics: Moving forward. PLoS Genet. 2018;14(6):e1007362.
Skvortsova K, Iovino N, Bogdanović O. Functions and mechanisms of epigenetic inheritance in animals. Nat Rev Mol Cell Biol. 2018;19(12):774–90.
Anvar Z, Chakchouk I, Demond H, Sharif M, Kelsey G, Van den Veyver IB. DNA Methylation Dynamics in the Female Germline and Maternal-Effect Mutations That Disrupt Genomic Imprinting. Genes (Basel). 2021;12(8):1214.
Marshall KL, Wang J, Ji T, Rivera RM. The effects of biological aging on global DNA methylation, histone modification, and epigenetic modifiers in the mouse germinal vesicle stage oocyte. Anim Reprod. 2018;15(4):1253–67.
Sun X, Lu J, Li H, Huang B. The Role of m(6)A on female reproduction and fertility: from gonad development to ovarian aging. Front Cell Dev Biol. 2022;10:884295.
Wang MK, Gao CC, Yang YG. Emerging Roles of RNA Methylation in Development. Acc Chem Res. 2023;56(23):3417–27.
Chang Y, Yi M, Wang J, Cao Z, Zhou T, Ge W, et al. Genetic Regulation of N6-Methyladenosine-RNA in Mammalian Gametogenesis and Embryonic Development. Front Cell Dev Biol. 2022;10:819044.
Liu H, Zheng J, Liao A. The regulation and potential roles of m6A modifications in early embryonic development and immune tolerance at the maternal-fetal interface. Front Immunol. 2022;13:988130.
Jiang X, Cheng Y, Zhu Y, Xu C, Li Q, **ng X, et al. Maternal NAT10 orchestrates oocyte meiotic cell-cycle progression and maturation in mice. Nat Commun. 2023;14(1):3729.
Ding C, Lu J, Li J, Hu X, Liu Z, Su H, et al. RNA-methyltransferase Nsun5 controls the maternal-to-zygotic transition by regulating maternal mRNA stability. Clin Transl Med. 2022;12(12):e1137.
Zhang M, Zhang S, Zhai Y, Han Y, Huang R, An X, et al. Cycloleucine negatively regulates porcine oocyte maturation and embryo development by modulating N6-methyladenosine and histone modifications. Theriogenology. 2022;179:128–40.
Kordowitzki P, Graczyk S, Haghani A, Klutstein M. Oocyte Aging: A Multifactorial Phenomenon in A Unique Cell. Aging Dis. 2024;15(1):5–21.
Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of Maternal Age on Oocyte and Embryo Competence. Front Endocrinol (Lausanne). 2018;9:327.
Ge ZJ, Schatten H, Zhang CL, Sun QY. Oocyte ageing and epigenetics. Reproduction. 2015;149(3):R103–14.
Qian Y, Tu J, Tang NL, Kong GW, Chung JP, Chan WY, et al. Dynamic changes of DNA epigenetic marks in mouse oocytes during natural and accelerated aging. Int J Biochem Cell Biol. 2015;67:121–7.
Castillo-Fernandez J, Herrera-Puerta E, Demond H, Clark SJ, Hanna CW, Hemberger M, et al. Increased transcriptome variation and localised DNA methylation changes in oocytes from aged mice revealed by parallel single-cell analysis. Aging Cell. 2020;19(12):e13278.
Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019;20(10):590–607.
Pollard CA, Jenkins TG. Epigenetic mechanisms within the sperm epigenome and their diagnostic potential. Best Pract Res Clin Endocrinol Metab. 2020;34(6):101481.
Miller JL, Grant PA. The role of DNA methylation and histone modifications in transcriptional regulation in humans. Subcell Biochem. 2013;61:289–317.
Potabattula R, Trapphoff T, Dittrich M, Fic K, Ptak GE, Dieterle S, et al. Ribosomal DNA methylation in human and mouse oocytes increases with age. Aging (Albany NY). 2022;14(3):1214–32.
Fan LH, Wang ZB, Li QN, Meng TG, Dong MZ, Hou Y, et al. Absence of mitochondrial DNA methylation in mouse oocyte maturation, aging and early embryo development. Biochem Biophys Res Commun. 2019;513(4):912–8.
Wen X, Hou Y, Zhou L, Fang X. LINC00969 inhibits proliferation with metastasis of breast cancer by regulating phosphorylation of PI3K/AKT and ILP2 expression through HOXD8. PeerJ. 2023;11:e16679.
Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20(2):74–88.
Kordowitzki P, Haghani A, Zoller JA, Li CZ, Raj K, Spangler ML, et al. Epigenetic clock and methylation study of oocytes from a bovine model of reproductive aging. Aging Cell. 2021;20(5):e13349.
Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183–203.
Ezine E, Lebbe C, Dumaz N. Unmasking the tumourigenic role of SIN1/MAPKAP1 in the mTOR complex 2. Clin Transl Med. 2023;13(10):e1464.
Córdoba-Jover B, Ribera J, Portolés I, Lecue E, Rodriguez-Vita J, Pérez-Sisqués L, et al. Tcf20 deficiency is associated with increased liver fibrogenesis and alterations in mitochondrial metabolism in mice and humans. Liver Int. 2023;43(8):1822–36.
Zhang Y, Sun Z, Jia J, Du T, Zhang N, Tang Y, et al. Overview of Histone Modification. Adv Exp Med Biol. 2021;1283:1–16.
Mei NH, Guo SM, Zhou Q, Zhang YR, Liu XZ, Yin Y, et al. H3K4 Methylation Promotes Expression of Mitochondrial Dynamics Regulators to Ensure Oocyte Quality in Mice. Adv Sci (Weinh). 2023;10(12):e2204794.
Shao GB, Wang J, Zhang LP, Wu CY, ** J, Sang JR, et al. Aging alters histone H3 lysine 4 methylation in mouse germinal vesicle stage oocytes. Reprod Fertil Dev. 2015;27(2):419–26.
Bilmez Y, Talibova G, Ozturk S. Expression of the histone lysine methyltransferases SETD1B, SETDB1, SETD2, and CFP1 exhibits significant changes in the oocytes and granulosa cells of aged mouse ovaries. Histochem Cell Biol. 2022;158(1):79–95.
He Y, Gao M, Yang W, Sun S, Wang Q, Gu L. Melatonin ameliorates histone modification disorders in mammalian aged oocytes by neutralizing the alkylation of HDAC1. Free Radic Biol Med. 2023;208:361–70.
Wu X, Wang S, Guo Y, Song S, Zeng S. KAT8 functions in redox homeostasis and mitochondrial dynamics during mouse oocyte meiosis progression. Faseb j. 2024;38(2):e23435.
Manosalva I, González A. Aging alters histone H4 acetylation and CDC2A in mouse germinal vesicle stage oocytes. Biol Reprod. 2009;81(6):1164–71.
He Y, Li X, Gao M, Liu H, Gu L. Loss of HDAC3 contributes to meiotic defects in aged oocytes. Aging Cell. 2019;18(6):e13036.
Colella M, Cuomo D, Peluso T, Falanga I, Mallardo M, De Felice M, et al. Ovarian Aging: Role of Pituitary-Ovarian Axis Hormones and ncRNAs in Regulating Ovarian Mitochondrial Activity. Front Endocrinol (Lausanne). 2021;12:791071.
Zhang-Sun ZY, Xu XZ, Escames G, Lei WR, Zhao L, Zhou YZ, et al. Targeting NR1D1 in organ injury: challenges and prospects. Mil Med Res. 2023;10(1):62.
Mihalas BP, Camlin NJ, Xavier MJ, Peters AE, Holt JE, Sutherland JM, et al. The small non-coding RNA profile of mouse oocytes is modified during aging. Aging (Albany NY). 2019;11(10):2968–97.
Wang TY, Zhang J, Zhu J, Lian HY, Yuan HJ, Gao M, et al. Expression profiles and function analysis of microRNAs in postovulatory aging mouse oocytes. Aging (Albany NY). 2017;9(4):1186–201.
Chawra HS, Agarwal M, Mishra A, Chandel SS, Singh RP, Dubey G, et al. MicroRNA-21’s role in PTEN suppression and PI3K/AKT activation: Implications for cancer biology. Pathol Res Pract. 2024;254:155091.
Ni Y, Yang Y, Ran J, Zhang L, Yao M, Liu Z, et al. miR-15a-5p inhibits metastasis and lipid metabolism by suppressing histone acetylation in lung cancer. Free Radic Biol Med. 2020;161:150–62.
Lim S, Lee DE, da MorenaSilva F, Koopmans PJ, Vechetti IJ Jr, von Walden F, et al. MicroRNA control of the myogenic cell transcriptome and proteome: the role of miR-16. Am J Physiol Cell Physiol. 2023;324(5):C1101–c9.
Samy AM, Kandeil MA, Sabry D, Abdel-Ghany AA, Mahmoud MO. Exosomal miR-122, miR-128, miR-200, miR-298, and miR-342 as novel diagnostic biomarkers in NAFL/NASH: Impact of LPS/TLR-4/FoxO3 pathway. Arch Pharm (Weinheim). 2024;357(4):e2300631.
Liu X, Cui H, Bai Q, Piao H, Song Y, Yan G. miR-128-3p alleviates airway inflammation in asthma by targeting SIX1 to regulate mitochondrial fission and fusion. Int Immunopharmacol. 2024;130:111703.
Liu WW, Zheng SQ, Li T, Fei YF, Wang C, Zhang S, et al. RNA modifications in cellular metabolism: implications for metabolism-targeted therapy and immunotherapy. Signal Transduct Target Ther. 2024;9(1):70.
Camaioni A, Ucci MA, Campagnolo L, De Felici M, Klinger FG. The process of ovarian aging: it is not just about oocytes and granulosa cells. J Assist Reprod Genet. 2022;39(4):783–92.
Kinnear HM, Tomaszewski CE, Chang AL, Moravek MB, Xu M, Padmanabhan V, et al. The ovarian stroma as a new frontier. Reproduction. 2020;160(3):R25–r39.
Chang CL. Facilitation of Ovarian Response by Mechanical Force-Latest Insight on Fertility Improvement in Women with Poor Ovarian Response or Primary Ovarian Insufficiency. Int J Mol Sci. 2023;24(19):14751.
Lliberos C, Liew SH, Zareie P, La Gruta NL, Mansell A, Hutt K. Evaluation of inflammation and follicle depletion during ovarian ageing in mice. Sci Rep. 2021;11(1):278.
Babayev E, Duncan FE. Age-associated changes in cumulus cells and follicular fluid: the local oocyte microenvironment as a determinant of gamete quality. Biol Reprod. 2022;106(2):351–65.
Taylor CT, Scholz CC. The effect of HIF on metabolism and immunity. Nat Rev Nephrol. 2022;18(9):573–87.
Lim M, Thompson JG, Dunning KR. Hypoxia and Reproductive Health: Hypoxia and ovarian function: follicle development, ovulation, oocyte maturation. Reproduction. 2021;161(1):F33–f40.
Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, et al. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003;9(11):639–43.
Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, et al. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod. 2006;12(11):655–60.
Yang L, Lin X, Tang H, Fan Y, Zeng S, Jia L, et al. Mitochondrial DNA mutation exacerbates female reproductive aging via impairment of the NADH/NAD(+) redox. Aging Cell. 2020;19(9):e13206.
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–33.
Sasaki H, Hamatani T, Kamijo S, Iwai M, Kobanawa M, Ogawa S, et al. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front Endocrinol (Lausanne). 2019;10:811.
Rodríguez-Varela C, Labarta E. Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review. Antioxidants (Basel). 2020;9(12):1197.
Lu X, Liu Y, Xu J, Cao X, Zhang D, Liu M, et al. Mitochondrial dysfunction in cumulus cells is related to decreased reproductive capacity in advanced-age women. Fertil Steril. 2022;118(2):393–404.
Buratini J, Dellaqua TT, Dal Canto M, La Marca A, Carone D, Mignini Renzini M, et al. The putative roles of FSH and AMH in the regulation of oocyte developmental competence: from fertility prognosis to mechanisms underlying age-related subfertility. Hum Reprod Update. 2022;28(2):232–54.
Babayev E, Wang T, Szigeti-Buck K, Lowther K, Taylor HS, Horvath T, et al. Reproductive aging is associated with changes in oocyte mitochondrial dynamics, function, and mtDNA quantity. Maturitas. 2016;93:121–30.
Meldrum DR, Casper RF, Diez-Juan A, Simon C, Domar AD, Frydman R. Aging and the environment affect gamete and embryo potential: can we intervene? Fertil Steril. 2016;105(3):548–59.
Park SU, Walsh L, Berkowitz KM. Mechanisms of ovarian aging. Reproduction. 2021;162(2):R19–r33.
Arbeithuber B, Cremona MA, Hester J, Barrett A, Higgins B, Anthony K, et al. Advanced age increases frequencies of de novo mitochondrial mutations in macaque oocytes and somatic tissues. Proc Natl Acad Sci U S A. 2022;119(15):e2118740119.
Zhang C, Dong X, Yuan X, Song J, Wang J, Yin X, et al. Post-ovulatory aging affects mitochondria, spindle and protein metabolism in mouse oocytes. Reproduction. 2023;166(6):473–84.
van der Reest J, Nardini Cecchino G, Haigis MC, Kordowitzki P. Mitochondria: Their relevance during oocyte ageing. Ageing Res Rev. 2021;70:101378.
Khan SA, Reed L, Schoolcraft WB, Yuan Y, Krisher RL. Control of mitochondrial integrity influences oocyte quality during reproductive aging. Mol Hum Reprod. 2023;29(9):gaad028.
** X, Wang K, Wang L, Liu W, Zhang C, Qiu Y, et al. RAB7 activity is required for the regulation of mitophagy in oocyte meiosis and oocyte quality control during ovarian aging. Autophagy. 2022;18(3):643–60.
Borcherding N, Brestoff JR. The power and potential of mitochondria transfer. Nature. 2023;623(7986):283–91.
Monzel AS, Enríquez JA, Picard M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat Metab. 2023;5(4):546–62.
Zhu Z, Xu W, Liu L. Ovarian aging: mechanisms and intervention strategies. Med Rev (2001). 2022;2(6):590–610.
Shen L, Liu J, Luo A, Wang S. The stromal microenvironment and ovarian aging: mechanisms and therapeutic opportunities. J Ovarian Res. 2023;16(1):237.
Metcalfe NB, Olsson M. How telomere dynamics are influenced by the balance between mitochondrial efficiency, reactive oxygen species production and DNA damage. Mol Ecol. 2022;31(23):6040–52.
Gao X, Yu X, Zhang C, Wang Y, Sun Y, Sun H, et al. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-related Diseases. Stem Cell Rev Rep. 2022;18(7):2315–27.
Bazzani V, EquisoainRedin M, McHale J, Perrone L, Vascotto C. Mitochondrial DNA Repair in Neurodegenerative Diseases and Ageing. Int J Mol Sci. 2022;23(19):11391.
Filograna R, Mennuni M, Alsina D, Larsson NG. Mitochondrial DNA copy number in human disease: the more the better? FEBS Lett. 2021;595(8):976–1002.
Kadenbach B, Münscher C, Frank V, Müller-Höcker J, Napiwotzki J. Human aging is associated with stochastic somatic mutations of mitochondrial DNA. Mutat Res. 1995;338(1–6):161–72.
Zhao Y, Liu B, Xu L, Yu S, Fu J, Wang J, et al. ROS-Induced mtDNA Release: The Emerging Messenger for Communication between Neurons and Innate Immune Cells during Neurodegenerative Disorder Progression. Antioxidants (Basel). 2021;10(12):1917.
Quan Y, **n Y, Tian G, Zhou J, Liu X. Mitochondrial ROS-Modulated mtDNA: A Potential Target for Cardiac Aging. Oxid Med Cell Longev. 2020;2020:9423593.
Willson JA, Arienti S, Sadiku P, Reyes L, Coelho P, Morrison T, et al. Neutrophil HIF-1α stabilization is augmented by mitochondrial ROS produced via the glycerol 3-phosphate shuttle. Blood. 2022;139(2):281–6.
Budani MC, Tiboni GM. Effects of Supplementation with Natural Antioxidants on Oocytes and Preimplantation Embryos. Antioxidants (Basel). 2020;9(7):612.
Timóteo-Ferreira F, Abreu D, Mendes S, Matos L, Rodrigues AR, Almeida H, et al. Redox imbalance in age-related ovarian dysfunction and perspectives for its prevention. Ageing Res Rev. 2021;68:101345.
Yang CX, Liu S, Miao JK, Mou Q, Liu XM, Wang PC, et al. CoQ10 improves meiotic maturation of pig oocytes through enhancing mitochondrial function and suppressing oxidative stress. Theriogenology. 2021;159:77–86.
Yang L, Wang H, Song S, Xu H, Chen Y, Tian S, et al. Systematic understanding of anti-aging effect of Coenzyme Q10 on oocyte through a network pharmacology approach. Front Endocrinol (Lausanne). 2022;13:813772.
Ceci R, Duranti G, Giuliani S, Rossi MN, Dimauro I, Sabatini S, et al. The Impact of Spermidine on C2C12 myoblasts proliferation, redox status and polyamines metabolism under H(2)O(2) exposure. Int J Mol Sci. 2022;23(19):10986.
Vrijsen S, Besora-Casals L, van Veen S, Zielich J, Van den Haute C, Hamouda NN, et al. ATP13A2-mediated endo-lysosomal polyamine export counters mitochondrial oxidative stress. Proc Natl Acad Sci USA. 2020;117(49):31198–207.
Xu TT, Li H, Dai Z, Lau GK, Li BY, Zhu WL, et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging. 2020;12(7):6401–14.
Jia H, Tang H, Wu W, Yan Z, Gao C, Gao L, et al. Putrescine alleviates the oxidative damage of cumulus-oocyte complex via improving fatty acid oxidation. Biochem Biophys Res Commun. 2023;684:149127.
Xu W, Li L, Sun J, Zhu S, Yan Z, Gao L, et al. Putrescine delays postovulatory aging of mouse oocytes by upregulating PDK4 expression and improving mitochondrial activity. Aging (Albany NY). 2018;10(12):4093–106.
Reiter RJ, Sharma R, Romero A, Manucha W, Tan DX, Zuccari D, et al. Aging-related ovarian failure and infertility: melatonin to the rescue. Antioxidants (Basel). 2023;12(3):695.
Qu J, Hu H, Niu H, Sun X, Li Y. Melatonin restores the declining maturation quality and early embryonic development of oocytes in aged mice. Theriogenology. 2023;210:110–8.
Zou H, Chen B, Ding D, Gao M, Chen D, Liu Y, et al. Melatonin promotes the development of immature oocytes from the COH cycle into healthy offspring by protecting mitochondrial function. J Pineal Res. 2020;68(1):e12621.
Zhan C, Cao X, Zhang T, Guo J, Xu G, Wang H, et al. Melatonin protects porcine oocyte from copper exposure potentially by reducing oxidative stress potentially through the Nrf2 pathway. Theriogenology. 2022;193:1–10.
Yang D, Mu Y, Wang J, Zou W, Zou H, Yang H, et al. Melatonin enhances the developmental potential of immature oocytes from older reproductive-aged women by improving mitochondrial function. Heliyon. 2023;9(9):e19366.
Liu H, An ZY, Li ZY, Yang LH, Zhang XL, Lv YT, et al. The ginsenoside Rh2 protects porcine oocytes against aging and oxidative stress by regulating SIRT1 expression and mitochondrial activity. Theriogenology. 2023;200:125–35.
Habibalahi A, Campbell JM, Bertoldo MJ, Mahbub SB, Goss DM, Ledger WL, et al. Unique deep radiomic signature Shows NMN treatment reverses morphology of oocytes from Aged Mice. Biomedicines. 2022;10(7):1544.
Li L, Han Q, Chen Y, Zhang M, Wang L, An X, et al. β-nicotinamide mononucleotide rescues the quality of aged oocyte and improves subsequent embryo development in pigs. PLoS ONE. 2023;18(10):e0291640.
Abbasi B, Dong Y, Rui R. Resveratrol hinders postovulatory aging by modulating oxidative stress in porcine oocytes. Molecules. 2021;26(21):6346.
Yin YJ, Zhang YH, Wang Y, Jiang H, Zhang JB, Liang S, et al. Ferulic acid ameliorates the quality of in vitro-aged bovine oocytes by suppressing oxidative stress and apoptosis. Aging. 2023;15:12497–512.
Tsui KH, Li CJ. Mitoquinone shifts energy metabolism to reduce ROS-induced oxeiptosis in female granulosa cells and mouse oocytes. Aging (Albany NY). 2023;15(1):246–60.
Zheng L, Luo Y, Zhou D, Liu H, Zhou G, Meng L, et al. Leonurine improves bovine oocyte maturation and subsequent embryonic development by reducing oxidative stress and improving mitochondrial function. Theriogenology. 2023;199:11–8.
Li C, Zhang H, Wu H, Li R, Wen D, Tang Y, et al. Intermittent fasting reverses the declining quality of aged oocytes. Free Radic Biol Med. 2023;195:74–88.
Lin PH, Su WP, Li CJ, Lin LT, Sheu JJ, Wen ZH, et al. Investigating the role of ferroptosis-related genes in ovarian aging and the potential for nutritional intervention. Nutrients. 2023;15(11):2461.
Kang B, Wang X, An X, Ji C, Ling W, Qi Y, et al. Polyamines in Ovarian Aging and Disease. Int J Mol Sci. 2023;24(20):15330.
Niu C, Jiang D, Guo Y, Wang Z, Sun Q, Wang X, et al. Spermidine suppresses oxidative stress and ferroptosis by Nrf2/HO-1/GPX4 and Akt/FHC/ACSL4 pathway to alleviate ovarian damage. Life Sci. 2023;332:122109.
Jiang D, Guo Y, Niu C, Long S, Jiang Y, Wang Z, et al. Exploration of the antioxidant effect of spermidine on the ovary and screening and identification of differentially expressed proteins. Int J Mol Sci. 2023;24(6):5793.
Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22(12):1428–38.
Uemura T, Higashi K, Takigawa M, Toida T, Kashiwagi K, Igarashi K. Polyamine modulon in yeast-Stimulation of COX4 synthesis by spermidine at the level of translation. Int J Biochem Cell Biol. 2009;41(12):2538–45.
Liu R, Li X, Ma H, Yang Q, Shang Q, Song L, et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic Biol Med. 2020;161:339–50.
Al-Habsi M, Chamoto K, Matsumoto K, Nomura N, Zhang B, Sugiura Y, et al. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science (New York, NY). 2022;378(6618):eabj3510.
Puleston DJ, Buck MD, Klein Geltink RI, Kyle RL, Caputa G, O’Sullivan D, et al. Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab. 2019;30(2):352–63.e8.
Barba-Aliaga M, Alepuz P. Role of eIF5A in Mitochondrial Function. Int J Mol Sci. 2022;23(3):1284.
Park MH, Wolff EC. Hypusine, a polyamine-derived amino acid critical for eukaryotic translation. J Biol Chem. 2018;293(48):18710–8.
Igarashi K, Kashiwagi K. Functional roles of polyamines and their metabolite acrolein in eukaryotic cells. Amino Acids. 2021;53(10):1473–92.
Pegg AE. The function of spermine. IUBMB Life. 2014;66(1):8–18.
Tassani V, Campagnolo M, Toninello A, Siliprandi D. The contribution of endogenous polyamines to the permeability transition of rat liver mitochondria. Biochem Biophys Res Commun. 1996;226(3):850–4.
Pirinen E, Kuulasmaa T, Pietilä M, Heikkinen S, Tusa M, Itkonen P, et al. Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism. Mol Cell Biol. 2007;27(13):4953–67.
Liu C, Perez-Leal O, Barrero C, Zahedi K, Soleimani M, Porter C, et al. Modulation of polyamine metabolic flux in adipose tissue alters the accumulation of body fat by affecting glucose homeostasis. Amino Acids. 2014;46(3):701–15.
Jell J, Merali S, Hensen ML, Mazurchuk R, Spernyak JA, Diegelman P, et al. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J Biol Chem. 2007;282(11):8404–13.
Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci USA. 2016;113(44):E6806–12.
Aye I, Gong S, Avellino G, Barbagallo R, Gaccioli F, Jenkins BJ, et al. Placental sex-dependent spermine synthesis regulates trophoblast gene expression through acetyl-coA metabolism and histone acetylation. Commun Biol. 2022;5(1):586.
Grancara S, Dalla Via L, García-Argáez AN, Ohkubo S, Pacella E, Manente S, et al. Spermine cycling in mitochondria is mediated by adenine nucleotide translocase activity: mechanism and pathophysiological implications. Amino Acids. 2016;48(10):2327–37.
Tao Y, Liu XJ. Deficiency of ovarian ornithine decarboxylase contributes to aging-related egg aneuploidy in mice. Aging Cell. 2013;12(1):42–9.
Tao Y, Tartia A, Lawson M, Zelinski MB, Wu W, Liu JY, et al. Can peri-ovulatory putrescine supplementation improve egg quality in older infertile women? J Assist Reprod Genet. 2019;36(3):395–402.
Liu XJ. Targeting oocyte maturation to improve fertility in older women. Cell Tissue Res. 2016;363(1):57–68.
Shi C, Yan Z, Zhang Y, Qin L, Wu W, Gao C, et al. Effects of putrescine on the quality and epigenetic modification of mouse oocytes during in vitro maturation. Reprod Fertil Dev. 2022;34(15):957–70.
Liu D, Mo G, Tao Y, Wang H, Liu XJ. Putrescine supplementation during in vitro maturation of aged mouse oocytes improves the quality of blastocysts. Reprod Fertil Dev. 2017;29(7):1392–400.
Zhang Y, Bai J, Cui Z, Li Y, Gao Q, Miao Y, et al. Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging. Nat Aging. 2023;3(11):1372–86.
Jiang D, Jiang Y, Long S, Chen Z, Li Y, Mo G, et al. Spermidine at supraphysiological doses induces oxidative stress and granulosa cell apoptosis in mouse ovaries. Theriogenology. 2021;168:25–32.
Miao Y, Cui Z, Gao Q, Rui R, **ong B. Nicotinamide Mononucleotide Supplementation Reverses the Declining Quality of Maternally Aged Oocytes. Cell Rep. 2020;32(5):107987.
Rai PK, Craven L, Hoogewijs K, Russell OM, Lightowlers RN. Advances in methods for reducing mitochondrial DNA disease by replacing or manipulating the mitochondrial genome. Essays Biochem. 2018;62(3):455–65.
Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350(9072):186–7.
Tesarik J, Nagy ZP, Sousa M, Mendoza C, Abdelmassih R. Fertilizable oocytes reconstructed from patient’s somatic cell nuclei and donor ooplasts. Reprod Biomed Online. 2001;2(3):160–4.
Morimoto Y, Gamage USK, Yamochi T, Saeki N, Morimoto N, Yamanaka M, et al. Mitochondrial transfer into human oocytes improved embryo quality and clinical outcomes in recurrent pregnancy failure cases. Int J Mol Sci. 2023;24(3):2738.
Zhang Q, Hao JX, Liu BW, Ouyang YC, Guo JN, Dong MZ, et al. Supplementation of mitochondria from endometrial mesenchymal stem cells improves oocyte quality in aged mice. Cell Prolif. 2023;56(3): e13372.
Wang ZB, Hao JX, Meng TG, Guo L, Dong MZ, Fan LH, et al. Transfer of autologous mitochondria from adipose tissue-derived stem cells rescues oocyte quality and infertility in aged mice. Aging. 2017;9(12):2480–8.
Zhang H, Fang Y, Gao Y, Zeng X, Lu Z, Liu L, et al. Brown adipose tissue-derived exosomes delay fertility decline in aging mice. Front Endocrinol (Lausanne). 2023;14:1180104.
Li J, Wang R, Chen Q, Tian Y, Gao L, Lei A. Salidroside improves porcine oocyte maturation and subsequent embryonic development by promoting lipid metabolism. Theriogenology. 2022;192:89–96.
Li CJ, Lin LT, Tsui KH. Dehydroepiandrosterone Shifts Energy Metabolism to Increase Mitochondrial Biogenesis in Female Fertility with Advancing Age. Nutrients. 2021;13(7):2449.
Hou HY, Wang X, Yu Q, Li HY, Li SJ, Tang RY, et al. Evidence that growth hormone can improve mitochondrial function in oocytes from aged mice. Reproduction. 2018;157(4):345–58.
Acknowledgements
Not applicable.
Funding
National Natural Science Foundation of China (No. 82271672).
The Key Research & Developmental Program of Hubei Province (2022BCA042).
The Interdisciplinary Innovative Talents Foundation from Renmin Hospital of Wuhan University (JCRCWL-2022–001).
Author information
Authors and Affiliations
Contributions
Bao S.L. contributed to the conceptualization of the idea of the review, drafted the manuscript, and edited all figures. Liu S. critically reviewed the manuscript. Yin T.L. approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bao, S., Yin, T. & Liu, S. Ovarian aging: energy metabolism of oocytes. J Ovarian Res 17, 118 (2024). https://doi.org/10.1186/s13048-024-01427-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-024-01427-y