Abstract
Pyroptosis is a cell death process characterized by cell swelling until membrane rupture and release of intracellular contents. As an effective tumor treatment strategy, inducing tumor cell pyroptosis has received widespread attention. In this process, the immune components within the tumor microenvironment play a key regulatory role. By regulating and altering the functions of immune cells such as cytotoxic T lymphocytes, natural killer cells, tumor-associated macrophages, and neutrophils, tumor cell pyroptosis can be induced. This article provides a comprehensive review of the molecular mechanisms of cell pyroptosis, the impact of the tumor immune microenvironment on tumor cell pyroptosis, and its mechanisms. It aims to gain an in-depth understanding of the communication between the tumor immune microenvironment and tumor cells, and to provide theoretical support for the development of new tumor immunotherapies.
Similar content being viewed by others
Introduction
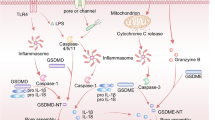
Pyroptosis, a form of programmed cell death, is closely connected to the inflammatory response, facilitating communication between innate and adaptive immunity [1, 2]. This emerging type of regulated cell death significantly influences cancer modulation, antitumor immunity, and the prognosis of cancer patients [2]. The impacts of pyroptosis are not only inhibiting tumor cell proliferation but also sha** an immunosuppressive microenvironment whichs promote tumor growth [3]. This immunosuppressive microenvironment has implications for the efficacy of anticancer therapy.
The tumor microenvironment (TME) comprises a diverse array of non-tumor cells, such as immune cells, stromal cells, and blood vessels, as well as structural components within the tumors, including extracellular matrix proteins and cytokines. These components interact with the tumor cells, collectively influencing tumor development, metastasis, and ultimately determining the tumor's responsiveness to various treatment strategies. Cancer cells often develop drug resistance due to genomic instability, while non-tumor cells in the TME are genetically more stable and respond better to therapies [5]. Resha** the TIME and restoring the tumor-killing ability of anti-tumor immune cells is a key area of research. Tumor immunotherapy, particularly chimeric antigen receptor (CAR)-T cell therapy and immune checkpoint inhibitors (ICIs), has shown promising results in combating tumor immune escape [6].
Pyroptosis is a critical factor in the origin, management, and outcome of tumors. Understanding the features and molecular mechanisms of cell pyroptosis, as well as the regulatory impact of TIME on tumor cell pyroptosis, is essential for advancing therapeutic approaches and improving treatment efficacy. This article aims to explore the characteristics and molecular mechanisms of pyroptosis, the influence of immune cells within the TIME on tumor cell pyroptosis, and two key tumor immunotherapy approaches. By gaining a comprehensive understanding of pyroptosis, this research aims to provide valuable insights for the development of new tumor immunotherapy strategies.
The characteristics and molecular mechanisms of cell pyroptosis
The emerging of pyroptosis
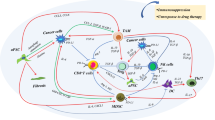
In the 1990s, pyroptosis was initially discovered in mouse macrophages infected by Shigella Flexner and was classified as apoptosis mistakenly [7, 8]. Subsequent research by Thirumalai et al. in 1997 revealed that Shigella dysenteriae activated caspase-1 in human monocyte-derived macrophages, leading to the maturation and subsequent release of Interleukin-1β (IL-1β) [9]. The Zychlinsky laboratory in 1999 demonstrated that knocking out caspase-1 could prevent cell death caused by Salmonella [10]. The term “pyroptosis”' was coined in 2001 by Cookson and Brennan, defining it as a caspase-1-dependent form of cell death distinct from apoptosis [11]. The concept of inflammasome activating inflammatory caspases and processing pro-IL-1β was introduced in 2002 [12]. In 2012, non-canonical caspase-11 was discovered to trigger cell death independently of caspase-1 during Salmonella infection [13]. (Fig. 1) gasdermin D (GSDMD) was redefined as the executioner of pyroptosis in 2015 [5, 14, 15]. Since then, other proteins in the gasdermin family have been found to mediate pyroptosis through caspase cleavage. Wang et al. and Rogers et al. demonstrated in 2017 that chemotherapeutic agents could induce pyroptosis by activating caspase-3 to cleave GSDME [16, 17]. The discovery has been widely utilized in tumor treatment. The Nomenclature Committee on Cell Death revised the definition of pyroptosis in 2018, describing it as a form of regulated cell death that critically depends on the formation of plasma membrane pores by members of the gasdermin protein family, often (but not always) as a consequence of inflammatory caspase activation. Notably, caspase-8 was found to participate in pyroptosis by activating GSDME in 2019 [18]. Furthermore, enzymes produced by immune cells have been identified as mediators of pyroptosis, working by recognizing and cleaving gasdermin proteins, thus shedding light on the intricate communication between the immune microenvironment and parenchymal cells. Reports from 2020 suggest that granzyme B (GzmB) can directly cleave GSDME, leading to the activation of pyroptosis and triggering the antitumor immune response [63]. Despite advancements in therapies such as CAR-T cell therapy and ICIs, there are still limitations in the treatment and prognosis of cancer patients due to tumor immune escape mechanisms.
CAR-T cell therapy
CAR-T cell therapy, a form of immunotherapy, involves genetically engineering T cells to target and kill tumors using antibody-derived CARs [104]. These modified T cells target inhibitory signaling molecules present in tumor cells [105]. Upon recognition of tumor-associated antigens by CARs, CAR-T cell activity is significantly increased. The process by which CAR-T cells induce tumor cell death through pyroptosis has been well documented in the granzyme pathway. Despite being a groundbreaking advancement in cancer treatment, CAR-T cell therapy encounters challenges related to efficacy, toxicity, side effects, etc. [106].
CAR-T therapy, a highly personalized form of immunotherapy, holds great promise for tumor treatment. It is characterized by superior cytotoxicity, persistence, and antigen recognition capabilities despite tumor-induced immunosuppressive influences [107, 108]. This therapy has demonstrated long-lasting antitumor immune responses in B-cell malignancies such as acute lymphoblastic leukemia, chronic lymphocytic leukemia, and non-Hodgkin’s lymphoma [109]. The positive outcomes of CAR-T therapy led to the US Food and Drug Administration (FDA) approval of anti-CD19 CAR-T cell therapy for B cell malignancies, marking a historic and unprecedented milestone [110].
Although CAR-T cell therapy play a non-negligible role in tumor treatment, various challenges hinder its therapeutic efficacy in both solid tumors and hematological malignancies. Further investigations are needed to address the toxicity and side effects associated with this therapy [106]. Major limitations include life-threatening toxicities, limited efficacy against solid tumors, resistance to B cell malignancies, antigen escape, limited persistence, poor trafficking, tumor infiltration, and as well as the presence of an immunosuppressive microenvironment [106]. Factors contributing to these limitations in solid tumor treatment include physical barriers hindering CAR-T cell entry, migration hindrance, recruitment of immunosuppressive cells, and sha** of an immunosuppressive environment [104]. CRS is a common immune-mediated toxicity characterized by fever, hypotension, and respiratory insufficiency due to elevated serum cytokine levels. Strategies such as knocking out GSDME, depleting macrophages, or inhibiting caspase-1 in mouse models have shown promise in mitigating CRS [63]. Research indicates CRS severity is correlated with GSDME and lactate dehydrogenase levels [63]. Therefore, it is crucial to not only consider the effect of CAR-T treatment but also monitor and manage the occurrence of CRS.
In order to advance therapeutic interventions, particularly in reducing drug resistance and minimizing toxic side effects, current research suggests that enhancing the efficacy of CAR-T anti-cancer therapy can be achieved through the choice of T-cell subpopulations and the modification of their nature [111]. This includes adjusting the ratio of helper T cells (CD4+T cells) to CD8+T cells in a patient-specific manner and modifying the differentiation status of T-cell modification [112]. To address potential toxic side effects, it is important to increase the selectivity of the isoform of the target antigen isoform to prevent CAR-T cells from attacking healthy tissue [111]. Although various strategies have been proposed to overcome the limitations of CAR-T therapy, many have not progressed to clinical trials. Therefore, further investigation into existing methodological approaches and the development of innovative strategies are essential to enhance anti-tumor activity and reduce toxicity.
Immune checkpoint inhibitors
ICIs, a prominent form of immunotherapy, have received significant attention as compelling treatment options [113]. They have emerged as potent therapeutic options for a wide array of solid tumors. Among immune checkpoint regulators, CTLA-4, PD-1, and PD-L1 are prominent, drawing substantial interest in the field of oncology as promising and powerful targets for cancer therapeutics [114]. As commonly understood, cancer cells utilize various mechanisms to evade the human immune system, including evading recognition by immune cells, enhancing resistance to apoptotic pathways, and creating immunosuppressive conditions [114]. Additionally, immune checkpoints are recognized as negative regulators of immune response and play a crucial role in preventing excessive peripheral tissue damage [115]. For example, the PD-1/PD-L1 system actively suppresses T lymphocyte proliferation, cytokine production, and cytotoxicity in cancer cells, leading to fatigue and apoptosis of tumor-specific T cells, allowing cancer cells to evade immune responses [116]. While the specific mechanisms of CTLA-4 activity remain unknown, it is postulated that its presence on the surface of T cells dampens T cell activation. This occurs through the active conveyance of inhibitory signals to T cells, achieved by outcompeting CD28 in binding CD80 and CD86 [117]. Targeting PD-1, PD-L1, or CTLA-4 effectively reverses the suppression of cytotoxic T lymphocytes, leading to the elimination of tumor cells by restoring T cell functionality. In immune-competent hosts, tumors evade immune surveillance during tumorigenesis. Blocking PD-1/PD-L1 enables T cells to enhance their growth, cytotoxicity, and infiltration into tumors, ultimately reducing tumorigenesis [118]. Currently, many drugs have been developed based on studies of ICIs, such as Ipilimumab Pembrolizumab, and Atezolizumab.
In 2011, Ipilimumab became the first FDA-approved ICI after successful trials in metastatic melanoma [119]. It's a human IgG1 monoclonal antibody that targets CTLA-4. Mechanistically, it blocks the interaction between CTLA-4 and CD86/CD80 on T cells or antigen-presenting cells [120]. This interference prevents the inhibitory signals of CTLA-4 and allows CD28 to bind with CD80/CD86, ultimately promoting T-cell activation [121].
Pembrolizumab, a clinically approved PD-1 inhibitor, represents a significant advance in the treatment of unresectable, advanced, and metastatic cancer. Its FDA approval marks an important milestone in improving in the treatment outcomes for these complex cancer [122, 123]. Pembrolizumab is known for its strong binding to PD-1 with low affinity for Fc receptors and complement [124]. Pembrolizumab has become the first immune checkpoint inhibitor approved for first-line treatment in several melanomas. By preventing the suppression and deactivation of immune cells, pembrolizumab has revolutionised melanoma treatment and offers a new approach to this challenging cancer [125]. Pembrolizumab has shown significant potential in clinical trials, particularly in patients with higher levels of PD-L1, and has been approved for the treatment of multiple cancers.
Atezolizumab, a human IgG1 monoclonal antibody, has the distinction of being the first FDA-approved PD-L1 inhibitor approved by the FDA. It is used in the treatment of various cancers,including urothelial carcinoma, triple negative breast cancer, non-small cell lung cancer, and small cell lung cancer [126]. Atezolizumab is a genetically engineered PD-L1 inhibitor with a modified Fc domain designed to reduce interactions with Fcγ receptors to decrease reducing traditional antibody-dependent cell-mediated cytotoxicity. This modification is intended to enhance the drug's therapeutic efficacy of the drug while minimising potential side effects related to immune system activation [127]. This Fc domain modification has been linked to the prevention of PD-L1 expression on immune cells, resulting in a more effective anti-tumor immune response [128].
While individual ICIs have shown efficacy in the fight against, there is a growing focus in clinical practice on using combination therapies to increase their pharmacological impact and reduce potential side effects. An example of this is the apexample of this umab not only as a stand-alone treatment but also in combination with Nivolumab, a PD-1 inhibitor [120]. This co-administration therapy has been approved for the treatment of unresectable (advanced) melanoma, renal cell carcinoma, and colorectal cancer with either high microsatellite instability (MSI-H) or mismatch repair-deficient (dMMR) status [120]. The simultaneous use of these ICIs is intended to synergistically improve the therapeutic response, and represents a significant advance in cancer treatment [120].
Enhancing pyroptosis in tumor immunotherapy
Although current tumor immunotherapy has shown significant success, it still faces challenges in achieving efficacy in most patients [129]. Take ICIs for example, many tumours respond poorly or not at all to ICIs, in part due to a lack of tumour-infiltrating lymphocytes (TILs) [130]. As a result, there is a pressing need for additional strategies to enhance antitumor immunity, such as converting these immunologically “cold” tumors into “hot” tumors [130]. In contrast to apoptosis, which tumor cells often resist, numerous studies suggest that harnessing pyroptosis in the tumor microenvironment can trigger a robust immune response, potentially offering more effective cancer therapy options and improving patient survival [129, 131, 132].
Pyroptosis is closely related to the immune system. On the one hand, pyroptosis can stimulate the immune system through by activating immune cells and immune factors [133]. Pyroptosis-produced cytokines can attract immune cells and ignite the immune system, potentially improving the efficacy of tumor immunotherapies [134]. On the other hand, immune cells like T cells and NK cells in the TIME can induce pyroptosis in tumor cells by releasing perforin and granzyme. Various therapeutic approaches can boost the immune system by inducing pyroptosis directly or indirectly [135]. Combining pyroptosis induction with ICIs has shown synergistic antitumor effects, even in ICI-resistant tumors [75]. However, inducing pyroptosis alone may not effectively inhibit tumors, highlighting the importance of combining pyroptosis inducers for cold tumors [75]. In CAR-T Cell Therapy, CAR-T cells induce pyroptosis in tumor cells by activating the caspase-3/GSDME pathway through GzmB release [19]. Nevertheless, pyroptosis is also linked to the toxicity and side effects of this therapy. Therefore, it is important to further investigate the role of pyroptosis in immunotherapy to optimize treatment efficacy and minimize associated toxicities and side effects.
Conclusions
Although considerable progress has been made in understanding the molecular mechanism of pyroptosis through intensive research, further investigations are needed to explore the signalling pathway, additional regulatory factors, functions of other GSDM family members, and the pathological implications of pyroptosis. The interaction between pyroptosis and tumors is intricate and multifaceted. On the one hand, pyroptosis inhibits tumor cell proliferation, invasion, and metastasis [136]. On the other hand, pyroptosis shapes an immunosuppressive microenvironment suitable for tumor cell growth to promote tumor growth. Moreover, the specific regulatory mechanisms of pyroptosis in different types of tumors and stages of tumor development remain unclear due to the complex nature of these relationships [137]. Furthermore, the regulation of tumor cell pyroptosis by various immune cells is complex and varies depending on the distribution of immune cells and subtypes within a specific tumor. This complexity highlights the need for in-depth studies to unravel the regulatory mechanisms of pyroptosis in specific tumors. Overall, tumor immunotherapy encounters numerous challenges.
In recent years, tumor immunotherapy has seen significant advance, particularly in CAR-T therapy and tumor ICIs. However, challenges such as the instability of CAR-T efficacy, toxic side effects, and tolerance issues hindered their its clinical progress. It is imperative to investigate existing strategies and develop innovative approaches to improve antitumor efficacy and minimize toxicity. Research on immune checkpoint inhibitors has also faced obstacles, despite recent achievements. The pace of research in this area has slowed, and the decrease in experimental patients poses a significant challenge to clinical trials involving ICIs.
Targeting pyroptosis as a novel therapeutic strategy for the development of anticancer drugs destined for clinical use is an intricate and labor-intensive journey. Crafting potent medications that precisely activate cell pyroptosis in human systems, while simultaneously adhering to rigorous safety testing protocols, continues to pose a formidable challenge within the realm of pharmaceutical research [75]. The integration of targeted therapies, whether as inducers or inhibitors of pyroptosis, with immunotherapy modalities holds immense promise in this endeavor. This multifaceted approach has the potential to unlock new frontiers in cancer treatment, providing patients with more effective and personalized therapeutic options [129]. Additionally, the synergistic benefit of combining chemotherapy and ICIs in cancer therapy has been widely reported, but the role of pyroptosis in chemotherapy toxicity requires further investigation [129]. Moreover, the DNA damage inflicted by radiotherapy can provoke cell pyroptosis via diverse signaling pathways, leading to significant antitumor efficacy when synergistically paired with immunotherapy [137,138,139]. This synergistic approach harnesses the power of both treatment modalities to achieve robust therapeutic outcomes. In essence, the synergistic alliance of targeted therapy, radiotherapy, and chemotherapy with immunotherapy holds immense potential in the realm of antitumor therapy. Nevertheless, the precise sequence and timing of these combined treatment modalities are pivotal considerations that significantly influence therapeutic efficacy and ultimately, patient prognosis [130].
Availability of data and materials
This article will be accessed on online upon publication.
References
Silva MT. Bacteria-induced phagocyte secondary necrosis as a pathogenicity mechanism. J Leukoc Biol. 2010;88(5):885–96.
Hsu SK, Li CY, Lin IL, Syue WJ, Chen YF, Cheng KC, et al. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics. 2021;11(18):8813–35.
Chen X, Zeh HJ, Kang R, Kroemer G, Tang D. Cell death in pancreatic cancer: from pathogenesis to therapy. Nat Rev Gastroenterol Hepatol. 2021;18(11):804–23.
**ao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. https://doi.org/10.1016/j.pharmthera.2020.107753.
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–71.
Szeto GL, Finley SD. Integrative approaches to cancer immunotherapy. Trends Cancer. 2019;5(7):400–10.
Monack DM, Raupach B, Hromockyj AE. Falkow Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93(18):9833–8.
Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–9.
Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1beta-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65(12):5165–70.
Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96(5):2396–401.
Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–4.
Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26.
Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490(7419):288–91.
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–92.
He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1βsecretion. Cell Res. 2015;25(12):1285–98.
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103.
Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8: 14128.
Newton K, Wickliffe KE, Maltzman A, Dugger DL, Reja R, Zhang Y, et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature. 2019;575(7784):679–82.
Zhang Z, Zhang Y, **a S, Kong Q, Li S, Liu X, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579(7799):415–20.
Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science(New York, NY). 2020;368(6494):eaaz7548.
Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science (New York, NY). 2018;362(6417):956–60.
Wei X, **e F, Zhou X, Wu Y, Yan H, Liu T, et al. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19(9):971–92.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–20.
Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–22.
Taabazuing CY, Griswold AR, Bachovchin DA. The NLRP1 and CARD8 inflammasomes. Immunol Rev. 2020;297(1):13–25.
Barnett KC, Li S, Liang K, Ting JP. A 360°view of the inflammasome: mechanisms of activation, cell death, and diseases. Cell. 2023;186(11):2288–312.
Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 2015;25(5):308–15.
Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR. Pattern recognition receptors and the host cell death molecular machinery. Front Immunol. 2018;9: 2379.
Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–32.
Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–87.
** T, Curry J, Smith P, Jiang J, **ao TS. Structure of the NLRP1 caspase recruitment domain suggests potential mechanisms for its association with procaspase-1. Proteins. 2013;81(7):1266–70.
Duncan JA, Canna SW. The NLRC4 inflammasome. Immunol Rev. 2018;281(1):115–23.
Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein Cell. 2014;5(1):12–20.
Johnson DC, Taabazuing CY, Okondo MC, Chui AJ, Rao SD, Brown FC, et al. DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat Med. 2018;24(8):1151–6.
Bauernfried S, Scherr MJ, Pichlmair A, Duderstadt KE, Hornung V. Human NLRP1 is a sensor for double-stranded RNA. Science (New York, NY). 2021;371(6528):eabd0811.
Yang J, Zhao Y, Shi J, Shao F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci USA. 2013;110(35):14408–13.
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–8.
Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med. 2015;21(8):906–13.
Xu H, Yang J, Gao W, Li L, Li P, Zhang L, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513(7517):237–41.
Wang Q, Gao H, Clark KM, Mugisha CS, Davis K, Tang JP, et al. CARD8 is an inflammasome sensor for HIV-1 protease activity. Sci (New York, NY). 2021;371(6535):eabe1707.
Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–14.
Fu J, Wu H. Structural mechanisms of NLRP3 Inflammasome assembly and activation. Annu Rev Immunol. 2023;41:301–16.
Mitoma H, Hanabuchi S, Kim T, Bao M, Zhang Z, Sugimoto N, et al. The DHX33 RNA helicase senses cytosolic RNA and activates the NLRP3 inflammasome. Immunity. 2013;39(1):123–35.
Huang X, Feng Y, **ong G, Whyte S, Duan J, Yang Y, et al. Caspase-11, a specific sensor for intracellular lipopolysaccharide recognition, mediates the non-canonical inflammatory pathway of pyroptosis. Cell Biosci. 2019;9:31.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–5.
Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–78.
Rühl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. European J Immunol. 2015;45(10):2927–36.
Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43(5):923–32.
Devant P, Dong Y, Mintseris J, Ma W, Gygi SP, Wu H, et al. Structural insights into cytokine cleavage by inflammatory caspase-4. Nature. 2023;624(7991):451–9.
Shi X, Sun Q, Hou Y, Zeng H, Cao Y, Dong M, et al. Recognition and maturation of IL-18 by caspase-4 noncanonical inflammasome. Nature. 2023;624(7991):442–50.
Exconde PM, Hernandez-Chavez C, Bourne CM, Richards RM, Bray MB, Lopez JL, et al. The tetrapeptide sequence of IL-18 and IL-1βregulates their recruitment and activation by inflammatory caspases. Cell Rep. 2023;42(12):113581.
Jiang M, Qi L, Li L, Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:112.
Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10(1):1689.
Akino K, Toyota M, Suzuki H, Imai T, Maruyama R, Kusano M, et al. Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer. Cancer Sci. 2007;98(1):88–95.
Kim MS, Chang X, Yamashita K, Nagpal JK, Baek JH, Wu G, et al. Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma. Oncogene. 2008;27(25):3624–34.
Kim MS, Lebron C, Nagpal JK, Chae YK, Chang X, Huang Y, et al. Methylation of the DFNA5 increases risk of lymph node metastasis in human breast cancer. Biochem Biophys Res Commun. 2008;370(1):38–43.
Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(46):E10888-e10897.
Hou J, Zhao R, **a W, Chang CW, You Y, Hsu JM, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22(10):1264–75.
Zheng M, Karki R, Vogel P, Kanneganti TD. Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell. 2020;181(3):674-87.e13.
Li X, Zhang T, Kang L, **n R, Sun M, Chen Q, et al. Apoptotic caspase-7 activation inhibits non-canonical pyroptosis by GSDMB cleavage. Cell Death Differ. 2023;30(9):2120–34.
Zhao Q, Chen DP, Chen HD, Wang YZ, Shi W, Lu YT, et al. NK-cell-elicited gasdermin-D-dependent hepatocyte pyroptosis induces neutrophil extracellular traps that facilitate HBV-related acute-on-chronic liver failure. Hepatology. 2024;0(0). https://doi.org/10.1097/HEP.0000000000000868.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science(New York,NY). 2018;359(6382):1361–5.
Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol. 2020;5(43):eaax7969.
Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–8.
Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nature Med. 2018;24(6):739–48.
Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity. 2018;48(4):812-30.e14.
Eckstein M, Gupta S. New insights in predictive determinants of the tumor immune microenvironment for immune checkpoint inhibition: a never ending story? Annals of translational medicine. 2019;7(Suppl 3):S135.
Jaime-Sánchez P, Catalán E, Uranga-Murillo I, Aguiló N, Santiago LP ML, et al. Antigen-specific primed cytotoxic T cells eliminate tumour cells in vivo and prevent tumour development, regardless of the presence of anti-apoptotic mutations conferring drug resistance. Cell Death Differ. 2018;25(9):1536–48.
Feng WQ, Zhang YC, Xu ZQ, Yu SY, Huo JT, Tuersun A, et al. IL-17A-mediated mitochondrial dysfunction induces pyroptosis in colorectal cancer cells and promotes CD8 + T-cell tumour infiltration. J Transl Med. 2023;21(1):335.
Karnkowska A, Vacek V, Zubáčová Z, Treitli SC, Petrželková R, Eme L, et al. A eukaryote without a mitochondrial organelle. Curr Biol. 2016;26(10):1274–84.
McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50(4):892–906.
Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23(7):1069–75.
Sivori S, Pende D, Quatrini L, Pietra G, Della Chiesa M, Vacca P, et al. NK cells and ILCs in tumor immunotherapy. Mol Aspects Med. 2021;80:100870.
Tsuchiya K. Switching from apoptosis to pyroptosis: gasdermin-elicited inflammation and antitumor immunity. Int J Mol Sci. 2021;22(1):426.
Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110.
Ma M, Wei N, Yang J, Ding T, Song A, Chen L, et al. Schisandrin B promotes senescence of activated hepatic stellate cell via NCOA4-mediated ferritinophagy. Pharm Biol. 2023;61(1):621–9.
Song A, Ding T, Wei N, Yang J, Ma M, Zheng S, et al. Schisandrin B induces HepG2 cells pyroptosis by activating NK cells mediated anti-tumor immunity. Toxicol Appl Pharmacol. 2023;472: 116574.
Bruno A, Mortara L, Baci D, Noonan DM, Albini A. Myeloid derived suppressor cells interactions with natural killer cells and pro-angiogenic activities: roles in tumor progression. Front Immunol. 2019;10:771.
Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–46.
Marofi F, Abdul-Rasheed OF, Rahman HS, Budi HS, Jalil AT, Yumashev AV, et al. CAR-NK cell in cancer immunotherapy a promising frontier. Cancer Sci. 2021;112(9):3427–36.
Mehta RS, Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol. 2018;9:283.
Ji X, Huang X, Li C, Guan N, Pan T, Dong J, et al. Effect of tumor-associated macrophages on the pyroptosis of breast cancer tumor cells. Cell Commun Signal. 2023;21(1):197.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416.
Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514.
**ang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Targeted Ther. 2021;6(1):75.
Zhou P, Guo H, Li Y, Liu Q, Qiao X, Lu Y, et al. Monocytes promote pyroptosis of endothelial cells during lung ischemia-reperfusion via IL-1R/NF-κB/NLRP3 signaling. Life Sci. 2021;276: 119402.
Prasad S, Ravindran J, Aggarwal BB. NF-kappa B and cancer: how intimate is this relationship? Mol Cell Biochem. 2010;336(1–2):25–37.
Li W, Zhu S, Li J, Assa A, Jundoria A, Xu J, et al. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem Pharmacol. 2011;81(9):1152–63.
Tang D, Kang R, 3rd HJ HJ, Lotze MT. High-mobility group box 1 and cancer. BBA. 2010;1799(1–2):131–40.
Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8(8):618–31.
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus"N2" TAN. Cancer Cell. 2009;16(3):183–94.
Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133(20):2159–67.
**a X, Zhang Z, Zhu C, Ni B, Wang S, Yang S, et al. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun. 2022;13(1):1017.
**ong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14(1):173.
Blaisdell A, Crequer A, Columbus D, Daikoku T, Mittal K, Dey SK, et al. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell. 2015;28(6):785–99.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-5. https://doi.org/10.1126/science.1092385.
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. el cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41.
Cools-Lartigue J, Spicer J, Najmeh S, Ferri L. Neutrophil extracellular traps in cancer progression. Cellular and molecular life sciences: CMLS. 2014;71(21):4179–94.
Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR1 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune Cytotoxicity. Immunity. 2020;52(5):856-71.e8.
Zhai R, Gong Z, Wang M, Ni Z, Zhang J, Wang M, et al. Neutrophil extracellular traps promote invasion and metastasis via NLRP3-mediated oral squamous cell carcinoma pyroptosis inhibition. Cell death discovery. 2024;10(1):214.
Thålin C, Hisada Y, Lundström S, Mackman N, Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler Thromb Vasc Biol. 2019;39(9):1724–38.
Almeida VH, Rondon AMR, Gomes T, Monteiro RQ. Novel aspects of extracellular vesicles as mediators of cancer-associated thrombosis. Cells. 2019;8(7):716.
Jiang X, Wang J, Deng X, **ong F, Ge J, **ang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18(1):10.
Liu G, Rui W, Zhao X, Lin X. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell Mol Immunol. 2021;18(5):1085–95.
Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257(1):107–26.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69.
Stancovski I, Schindler DG, Waks T, Yarden Y, Sela M, Eshhar Z. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J Immunol (Baltimore, Md:1950). 1993;151(11):6577–82.
Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):720–4.
Schubert ML, Hückelhoven A, Hoffmann JM, Schmitt A, Wuchter P, Sellner L, et al. Chimeric antigen receptor T cell therapy targeting CD19-positive leukemia and lymphoma in the context of stem cell transplantation. Human gene therapy. 2016;27(10):758–71.
Braendstrup P, Levine BL, Ruella M. The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19. Cytotherapy. 2020;22(2):57–69.
Skurikhin E, Pershina O, Zhukova M, Widera D, Ermakova N, Pan E, et al. Potential of stem cells and CART as a Potential polytherapy for small cell lung cancer. Front Cel Develop Biol. 2021;9.
Stock S, Schmitt M, Sellner L. Optimizing manufacturing protocols of chimeric antigen receptor t cells for improved anticancer immunotherapy. Int J Mol Sci. 2019;20(24):6223.
Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discovery. 2014;13(12):883–4.
Robert C. ade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801.
Abdkarimi S, Razi Soofiyani S, Elham G, Mashhadi Abdolahi H, Safarzadeh E, Baradaran B. Targeting immune checkpoints: building better therapeutic puzzle in pancreatic cancer combination therapy. Eur J Cancer Care. 2020;29(5):e13268.
Liu D, Gao S, Zhai Y, Yang X, Zhai G. Research progress of tumor targeted drug delivery based on PD-1/PD-L1. Int J Pharm. 2022;616:121527.
Pandey P, Khan F, Qari HA, Upadhyay TK, Alkhateeb AF, Oves M. Revolutionization in cancer therapeutics via targeting major immune checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals (Basel, Switzerland). 2022;15(3):335.
Zandberg DP, Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50(7):627–32.
Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21(7):509–28.
Klein O, Kee Markman B, Carlino MS, Underhill C, Palmer J, et al. Evaluation of TMB as a predictive biomarker in patients with solid cancers treated with anti-PD-1/CTLA-4 combination immunotherapy. Cancer Cell. 2021;39(5):592–3.
Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–30.
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet (London, England). 2019;394(10212):1915–28.
Nassar AH, Adib E, Abou Alaiwi S, El Zarif T, Groha S, Akl EW, et al. Ancestry-driven recalibration of tumor mutational burden and disparate clinical outcomes in response to immune checkpoint inhibitors. Cancer Cell. 2022;40(10):1161-72.e5.
Foord E, Klynning C, Schoutrop E, Förster JM, Krieg J, Mörtberg A, et al. Profound functional suppression of tumor-infiltrating t-cells in ovarian cancer patients can be reversed using PD-1-blocking antibodies or DARPin®proteins. J Immunol Res. 2020;2020:7375947.
Leonetti A, Wever B, Mazzaschi G, Assaraf YG, Rolfo C, Quaini F, et al. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2019;46: 100644.
Markham A. Atezolizumab: first global approval. Drugs. 2016;76(12):1227–32.
Shah NJ, Kelly WJ, Liu SV, Choquette K, Spira A. Product review on the Anti-PD-L1 antibody atezolizumab. Hum Vaccin Immunother. 2018;14(2):269–76.
Baker M, Cordes L, Brownell I. Avelumab: a new standard for treating metastatic merkel cell carcinoma. Expert Rev Anticancer Ther. 2018;18(4):319–26.
Zhang Z, Zhang Y, Lieberman J. Lighting a fire: can we harness pyroptosis to ignite antitumor immunity? Cancer Immunol Res. 2021;9(1):2–7.
Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7(1):196.
Dasgupta A, Nomura M, Shuck R, Yustein J. Cancer’s Achilles’ heel: apoptosis and necroptosis to the rescue. Int J Mol Sci. 2016;18(1):23.
Huang X, **ao F, Li Y, Qian W, Ding W, Ye X. Bypassing drug resistance by triggering necroptosis: recent advances in mechanisms and its therapeutic exploitation in leukemia. Journal of experimental&clinical cancer research: CR. 2018;37(1):310.
Minton K. Pyroptosis heats tumour immunity. Nat Rev Immunol. 2020;20(5):274–5.
Li L, Jiang M, Qi L, Wu Y, Song D, Gan J, et al. Pyroptosis, a new bridge to tumor immunity. Cancer Sci. 2021;112(10):3979–94.
Okondo MC, Johnson DC, Sridharan R, Go EB, Chui AJ, Wang MS, et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 2017;13(1):46–53.
Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6(1):128.
Philippou Y, Sjoberg H, Lamb AD, Camilleri P, Bryant RJ. Harnessing the potential of multimodal radiotherapy in prostate cancer. Nat Rev Urol. 2020;17(6):321–38.
McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20(4):203–17.
Liu YG, Chen JK, Zhang ZT, Ma XJ, Chen YC, Du XM, et al. NLRP3 inflammasome activation mediates radiation-induced pyroptosis in bone marrow-derived macrophages. Cell death&disease. 2017;8(2): e2579.
Acknowledgements
We would like to express our gratitude to the National Natural Science Foundation of China for their support, and to Dr. HZ for his guidance and funding in this article.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC) Regional Fund (Grant No.82360535) and Yunnan Provincial Department of Education Science Research Fund Project (Grant No.2024J0019).
Author information
Authors and Affiliations
Contributions
MH, FD, and XS Wrote the original draft and the revised manuscript. FY and HZ contributed to the conceptualization and funding acquisition, and provided guidance for the revision of the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed to the publication of the article to the journal.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, M., Deng, F., Song, X. et al. The crosstalk between immune cells and tumor pyroptosis: advancing cancer immunotherapy strategies. J Exp Clin Cancer Res 43, 190 (2024). https://doi.org/10.1186/s13046-024-03115-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-024-03115-7