Abstract
Background
Colorectal carcinoma (CRC) is the third most common cancer and second most common cause of cancer-related deaths worldwide. Ribonucleic acid (RNA) N6-methyladnosine (m6A) and methyltransferase-like 3 (METTL3) play key roles in cancer progression. However, the roles of m6A and METTL3 in CRC progression require further clarification.
Methods
Adenoma and CRC samples were examined to detect m6A and METTL3 levels, and tissue microarrays were performed to evaluate the association of m6A and METTL3 levels with the survival of patients with CRC. The biological functions of METTL3 were investigated through cell counting kit-8, wound healing, and transwell assays. M6A epitranscriptomic microarray, methylated RNA immunoprecipitation-qPCR, RNA stability, luciferase reporter, and RNA immunoprecipitation assays were performed to explore the mechanism of METTL3 in CRC progression.
Results
M6A and METTL3 levels were substantially elevated in CRC tissues, and patients with CRC with a high m6A or METTL3 levels exhibited shorter overall survival. METTL3 knockdown substantially inhibited the proliferation, migration, and invasion of CRC cells. An m6A epitranscriptomic microarray revealed that the cell polarity regulator Crumbs3 (CRB3) was the downstream target of METTL3. METTL3 knockdown substantially reduced the m6A level of CRB3, and inhibited the degradation of CRB3 mRNA to increase CRB3 expression. Luciferase reporter assays also showed that the transcriptional level of wild-type CRB3 significantly increased after METTL3 knockdown but not its level of variation. Knockdown of YT521-B homology domain–containing family protein 2 (YTHDF2) substantially increased CRB3 expression. RNA immunoprecipitation assays also verified the direct interaction between the YTHDF2 and CRB3 mRNA, and this direct interaction was impaired after METTL3 inhibition. In addition, CRB3 knockdown significantly promoted the proliferation, migration, and invasion of CRC cells. Mechanistically, METTL3 knockdown activated the Hippo pathway and reduced nuclear localization of Yes1-associated transcriptional regulator, and the effects were reversed by CRB3 knockdown.
Conclusions
M6A and METTL3 levels were substantially elevated in CRC tissues relative to normal tissues. Patients with CRC with high m6A or METTL3 levels exhibited shorter overall survival, and METTL3 promoted CRC progression. Mechanistically, METTL3 regulated the progression of CRC by regulating the m6A–CRB3–Hippo pathway.
Similar content being viewed by others
Background
Colorectal carcinoma (CRC) is the third most common cancer and second most common cause of cancer-related deaths worldwide [1]. Because of tumor metastasis and other complications, the mortality rate of CRC remains high. Therefore, a timely clarification of the molecular mechanism and effective therapeutic targets of CRC is warranted.
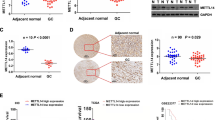
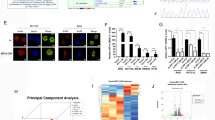
Ribonucleic acid (RNA) N6-methyladnosine (m6A) is the most common and abundant RNA modification in eukaryotes [2, 3]. M6A modification is mainly mediated by m6A methyltransferases, demethylases, and reader proteins. M6A methyltransferases mainly comprise methyltransferase-like 3 (METTL3) [4], methyltransferase-like 14 (METTL14) [5], Wilms tumor 1–associated protein (WTAP) [6], RNA binding motif protein 15 [7], and vir-like m6A methyltransferase associated [8]. M6A demethylases mainly comprise fat-mass and obesity-associated protein (FTO) [9] and alkylation repair homolog protein 5 (ALKBH5) [10]. Reader proteins mainly comprise YT521-B homology domain–containing family protein 1/2/3 (YTHDF1/2/3), YT521-B homology domain-–containing 1/2, insulin-like growth factor 2 mRNA binding proteins 1/2/3, and heterogeneous nuclear ribonucleoprotein family [11,12,13]. Several studies have verified that m6A modification plays a key role in cancer progression [9, 14]. METTL3 and METTL14 can regulate the progression of multiple types of cancer, including bladder cancer [15, 21]. FTO plays a central role in oral squamous cell carcinoma [22], hepatocellular carcinoma [5a), and a qPCR analysis indicated a substantial increase in CRB3 level after METTL3 knockdown (Fig. 5b and c). The results obtained from The Cancer Genome Atlas (TCGA) database revealed a substantial downregulation of CRB3 level in the CRC group (Additional file 2: Fig. S2b). A survival analysis indicated that the patients with CRC with high CRB3 level exhibited higher overall survival and disease-free survival relative to those without high CRB3 level (Additional file 2: Fig. S2c). Although METTL3 knockdown also promoted bridging integrator 1 (BIN1) expression, a substantial increase in BIN1 expression was observed in the CRC group (Additional file 2: Fig. S2a and 2d). The survival analysis did not indicate a correlation between BIN1 level and survival in patients with CRC (Additional file 2: Fig. S2e). In addition, the m6A level of CRB3 was substantially reduced after METTL3 knockdown (Fig. 5d). Luciferase reporters were revealed to determine the effect of m6A modification on CRB3 expression. For the variant form of CRB3, the adenosine bases in m6A consensus sequences (GGAC) were replaced by cytosine; thus, m6A modification was abolished. The results indicated that the transcriptional level of wild-type CRB3 significantly increased after METTL3 knockdown but not its level of variation (Fig. 5e). An RNA stability assay revealed that METTL3 knockdown substantially inhibited the degradation of CRB3 mRNA (Fig. 5f). Moreover, data from the TCGA database indicated that METTL3 mRNA expression in CRC tissues was negatively associated with CRB3 levels (Fig. 5g). Studies have reported that YTHDF2 can target mRNAs by recognizing m6A motif in CRC [5, 12]; thus, we explored the effect of YTHDF2 on CRB3. The results indicated that the expression of YTHDF2 was also significantly higher in adenoma and CRC tissues relative to normal tissues (Additional file 3: Fig. S3), and YTHDF2 knockdown substantially increased the level of CRB3 (Fig. 5h and i). RIP assays also verified the direct interaction between the YTHDF2 and CRB3 mRNA, and this direct interaction was impaired after METTL3 inhibition in SW620 and HCT116 cells (Fig. 5j and k). The results indicated that METTL3 regulated the expression of CRB3 in an m6A-YTHDF2-dependent manner.
Methyltransferase-like 3 regulated the expression of crumbs3 in an N6-methyladnosine -dependent manner. a Overlap** differentially N6-methyladnosine (m6A)-methylated genes between m6A methylation level and quantity level as filtered through a Venn diagram; b Crumbs3 (CRB3) mRNA level as measured after methyltransferase-like 3 (METTL3) knockdown; c CRB3 protein level as measured after METTL3 knockdown; d The m6A level of CRB3 as measured after METTL3 knockdown; e Luciferase reporters performed to determine the effect of m6A modification on CRB3 expression; f CRB3 mRNA expression as detected with or without treatment of actinomycin D at indicated time points; g The correlation between METTL3 and CRB3 expression in The Cancer Genome Atlas database for CRC; h CRB3 mRNA level as measured after YTH domain–containing family protein 2 (YTHDF2) knockdown; i CRB3 protein level as measured after YTHDF2 knockdown; j-k The direct interaction was verified between the YTHDF2 and CRB3 mRNA. Data are presented as means ± standard deviations (SDs). *P < 0.05, **P < 0.01, ***P < 0.001
CRB3 inhibited CRC proliferation and invasion
Our results also indicated that CRB3 level was substantially lower in the adenoma and CRC groups than in the normal group, which was consistent with the data from TCGA database (Fig. 6a and b, Additional file 2: Fig. S2b). To further investigate the function of CRB3 in CRC, CRB3 knockdown was performed in HCT116 and SW620 cells. CRB3 knockdown significantly promoted the proliferation, migration and invasion of HCT116 and SW620 cells (Fig. 6c-e). CRB3 knockdown also significantly increased the migration speed of HCT116 and SW620 cells (Fig. 6f and g). These results verified that CRB3 regulated CRC progression.
Crumbs3 inhibited colorectal carcinoma proliferation and invasion. Crumbs3 (CRB3) expression in both adenoma and colorectal carcinoma (CRC) as assayed through (a) qPCR and (b) Immunofluorescence; c Proliferation of HCT116 and SW620 cells as measured after CRB3 knockdown; Transwell assays performed with CRB3 knockdown in (d) HCT116 cells and (e) SW620 cells; Wound healing assay performed with CRB3 knockdown in (f) HCT116 cells and (g) SW620 cells. Data are presented as means ± standard deviations (SD). *P < 0.05, **P < 0.01, ***P < 0.001
METTL3 regulated CRC proliferation and invasion by CRB3-hippo pathway
Studies have demonstrated that CRB3 can regulate the Hippo pathway [30, 31]. In the present study, we discovered that CRB3 knockdown reduced MST1, LATS1, MOB1, and YAP phosphorylation levels, and it reduced the SAV1 levels in HCT116 and SW620 cells (Fig. 7a and b). Conversely, METTL3 knockdown substantially increased MST1, LATS1, MOB1, and YAP phosphorylation levels in HCT116 and SW620 cells (Fig. 7c and d). Studies have demonstrated that YAP enters the nucleus and acts as an oncogene; thus, we detected the level of YAP in nuclei. The results indicated that CRB3 knockdown markedly increased YAP protein levels in the nuclei of HCT116 and SW620 cells (Fig. 7e and f); however, METTL3 knockdown substantially reduced the YAP protein levels in the nuclei of HCT116 and SW620 cells (Fig. 7g and h).
Methyltransferase-like 3 and crumbs3 could both regulate Hippo pathway. Hippo pathway as detected after (a, b) Crumbs3 (CRB3) knockdown or (c, d) Methyltransferase-like 3 (METTL3) knockdown in HCT116 and SW620 cells; Yes1-associated transcriptional regulator (YAP) protein level as detected after (e, f) CRB3 knockdown or (g, h) METTL3 knockdown in nuclei of HCT116 and SW620 cells. Data are presented as means ± standard deviations (SD). *P < 0.05, **P < 0.01, ***P < 0.001
To test whether CRB3 functions downstream of METTL3, we knocked down CRB3 in the METTL3 knockdown background. The results revealed that the effects of METTL3 knockdown on cell proliferation, migration, and invasion were rescued by the CRB3 knockdown (Fig. 8a-d). In addition, CRB3 knockdown reduced the MST1, LATS1, MOB1, and YAP phosphorylation levels caused by METTL3 knockdown, indicating that CRB3 knockdown repressed activation of hippo pathway that was caused by METTL3 knockdown (Fig. 8e and f).
The effect of methyltransferase-like 3 on colorectal carcinoma progression was rescued by crumbs3. The proliferation of HCT116 (a) and SW620 cells (b) was rescued after crumbs3 (CRB3) knockdown; Migration and invasion was rescued after CRB3 knockdown in HCT116 (c) and SW620 cells (d); The activation of hippo pathway was reversed after CRB3 knockdown in HCT116 (e) and SW620 cells (f). Data are presented as means ± standard deviations (SD). *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
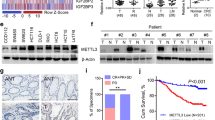
The incidence of CRC, which is a malignant tumor, has increased. The 5-year survival rate of patients with CRC is 65% [32], but it is extremely low in advanced-stage CRC. Therefore, techniques that aid the implementation of treatment strategies for CRC are urgently required because they can improve patient survival [27]. Studies have reported that m6A modification plays a key role in CRC [4, 5, 12, 33]. However, the changes to m6A in both adenoma and CRC are still unknown. In addition, the relationship between m6A level and the survival of patients with CRC requires further clarification. In the present study, we discovered that m6A levels are significantly increased in both adenoma and CRC tissues, indicating that m6A modification may be involved in the adenoma-to-CRC transition. A further examination indicated that the patients with CRC with high m6A level exhibited shorter overall survival.
M6A modification is mainly mediated by m6A methyltransferases, demethylases and reader proteins, and it regulates pre-mRNA splicing, miRNA processing, translation, and mRNA decay [34]. In the present study, we discovered that the protein level of METTL3 and m6A methylase activity were both significantly increased in CRC, indicating that METTL3 could be involved in the CRC progression. Furthermore, patients with CRC with high METTL3 level exhibited shorter overall survival, suggesting that METTL3 can also serve as a prognostic marker of CRC. These results are consistent with those reported by other studies [4, 35]. Subsequently, we verified the function of METTL3 in CRC. METTL3 knockdown inhibited the proliferation of HCT116 and SW620 cells, and it also substantially inhibited the migration and invasion of HCT116 and SW620 cells. These results indicated that METTL3 acts as an oncogene that promotes the progression of CRC. Studies have also reported on the key role of METTL3 in various cancers. METTL3 can regulate MALAT1 stabilization through m6A modification, and it activates NF-κB activity to promote the malignant progression of glioma [36]. METTL3 increases miR-1246 levels through m6A modification, thereby promoting non-small-cell lung cancer progression [37]. Moreover, METTL3 regulates the m6A modification of SPHK2 to promote the progression of gastric cancer [38]. These findings verified the role of METTL3 in cancers such as CRC. Therefore, METTL3 can be a new treatment target for cancers.
Through an m6A epitranscriptomic microarray analysis, we revealed that CRB3 might be the downstream target of METTL3. METTL3 knockdown substantially reduced the m6A level of CRB3 and inhibited the degradation of CRB3 mRNA, finally to increase CRB3 expression. Studies have reported that the m6A consensus sequences are GGAC [5]. Our study revealed that METTL3 knockdown increased the transcriptional level of CRB3. When the adenosine bases of GGAC in CRB3 were replaced by cytosine, the transcriptional level of CRB3 did not change. In addition, YTHDF2 could also regulate the CRC progression in an m6A-dependent manner [5, 12, 39]. In the present study, we discovered that YTHDF2 knockdown substantially increased the level of CRB3. The direct interaction between the YTHDF2 and CRB3 mRNA was also verified, and this direct interaction was impaired after METTL3 inhibition. These results indicated that METTL3 regulated the expression of CRB3 in an m6A-YTHDF2-dependent manner. CRB3 is a protein of cell polarity, and it is associated with contact inhibition [40]. Studies have demonstrated that CRB3 plays a central role in cancers such as CRC [30, 41, 42]. In the present study, we discovered that CRB3 levels in both adenoma and CRC were substantially lower than in normal tissues, and we also revealed that patients with CRC with high CRB3 level exhibited higher overall survival and disease-free survival. CRB3 knockdown significantly promoted the proliferation, migration, and invasion of HCT116 and SW620 cells. These results indicated that CRB3 regulates CRC progression.
The depletion of CRB3 can inhibit the Hippo pathway and lead to increased nuclear localization of YAP [30, 31, 43]. The Hippo pathway plays a crucial role in regulating CRC progression [44,45,46,47]. In the present study, we also observed that CRB3 knockdown inhibited the Hippo pathway and increased the nuclear localization of YAP, suggesting that CRB3 regulates CRC progression through the Hippo pathway. Conversely, METTL3 knockdown activated the Hippo pathway and reduced the nuclear localization of YAP. Finally, our results revealed that the effects of METTL3 knockdown on cell proliferation, migration, and invasion were rescued by the CRB3 knockdown. CRB3 knockdown reversed the activation of hippo pathway caused by METTL3 knockdown. Therefore, our study indicated that METTL3 facilitated CRC progression by regulating the m6A-CRB3-Hippo pathway, which is a novel mechanism for regulating CRC. Even though we demonstrated the regulatory mechanism of METTL3 in CRC, further studies are required. First, although a study reported that the selective first-in-class catalytic inhibitor of METTL3 (i.e., STM2457) can be used in treatment strategies for acute myeloid leukemia [48], the inhibitor of METTL3 has not yet been identified for the treatment of CRC. Therefore, further studies are required to identify the inhibitor of METTL3. Second, we discovered substantially elevated m6A levels in both adenoma and CRC; METTL3 level was substantially elevated in only CRC, not in adenoma. This suggests that other enzymes may also be involved in the m6A modification in adenoma, and further clarification was required.
Conclusion
In summary, we demonstrated that m6A and METTL3 levels are significantly increased in CRC. Patients with CRC with high m6A or METTL3 levels exhibit shorter overall survival, and METTL3 promoted CRC progression. Mechanistically, METTL3 regulates the progression of CRC by regulating the m6A–CRB3–Hippo pathway. These findings provide a new perspective for the implementation of targeted therapy for CRC.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRC:
-
Colorectal carcinoma
- RNA:
-
Ribonucleic acid
- m6A:
-
N6-methyladnosine
- METTL3:
-
Methyltransferase-like 3
- CRB3:
-
Crumbs3
- METTL14:
-
Methyltransferase-like 14
- WTAP:
-
Wilms tumor 1–associated protein
- FTO:
-
Fat-mass and obesity-associated protein
- ALKBH5:
-
Alkylation repair homolog protein 5
- YTHDF1/2/3:
-
YTH domain–containing family protein 1/2/3
- Normal:
-
Adjacent normal tissues
- IHC:
-
Immunohistochemistry
- TMA:
-
Tissue microarrays
- FBS:
-
Fetal bovine serum
- CCK8:
-
Cell counting kit-8
- qPCR:
-
Quantitative real-time polymerase chain reaction
- MST1:
-
Macrophage stimulating 1
- SAV1:
-
Salvador family WW domain containing protein 1
- LATS1:
-
Large tumor suppressor kinase 1
- MOB1:
-
MOB kinase activator 1
- YAP:
-
Yes1-associated transcriptional regulator
- RIP:
-
RNA immunoprecipitation
- DMS:
-
Differentially m6A-methylated sites
- DMG:
-
Differentially m6A-methylated genes
- TCGA:
-
The Cancer Genome Atlas
- BIN1:
-
Bridging integrator 1
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149(7):1635–46.
Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18(1):31–42.
Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN, et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18(1):112.
Chen X, Xu M, Xu X, Zeng K, Liu X, Pan B, et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19(1):106.
Selberg S, Blokhina D, Aatonen M, Koivisto P, Siltanen A, Mervaala E, et al. Discovery of small molecules that activate RNA methylation through cooperative binding to the METTL3-14-WTAP complex active site. Cell Rep. 2019;26(13):3762–3771 e3765.
Wang X, Tian L, Li Y, Wang J, Yan B, Yang L, et al. RBM15 facilitates laryngeal squamous cell carcinoma progression by regulating TMBIM6 stability through IGF2BP3 dependent. J Exp Clin Cancer Res. 2021;40(1):80.
Barros-Silva D, Lobo J, Guimaraes-Teixeira C, Carneiro I, Oliveira J, Martens-Uzunova ES, et al. VIRMA-dependent N6-Methyladenosine modifications regulate the expression of long non-coding RNAs CCAT1 and CCAT2 in prostate Cancer. Cancers (Basel). 2020;12(4):771.
Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-Methyladenosine RNA Demethylase. Cancer Cell. 2017;31(1):127–41.
Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, et al. M(6) a Demethylase ALKBH5 maintains Tumorigenicity of Glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31(4):591–606 e596.
Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20(3):285–95.
Zhou D, Tang W, Xu Y, Xu Y, Xu B, Fu S, et al. METTL3/YTHDF2 m6A axis accelerates colorectal carcinogenesis through epigenetically suppressing YPEL5. Mol Oncol. 2021;15(8):2172–84.
Ma L, Zhang X, Yu K, Xu X, Chen T, Shi Y, et al. Targeting SLC3A2 subunit of system XC(−) is essential for m(6) a reader YTHDC2 to be an endogenous ferroptosis inducer in lung adenocarcinoma. Free Radic Biol Med. 2021;168:25–43.
Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67(6):2254–70.
Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18(1):110.
Cheng M, Sheng L, Gao Q, **ong Q, Zhang H, Wu M, et al. The m(6) a methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF-kappaB/MYC signaling network. Oncogene. 2019;38(19):3667–80.
Wang Q, Chen C, Ding Q, Zhao Y, Wang Z, Chen J, et al. METTL3-mediated m(6) a modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2020;69(7):1193–205.
Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, et al. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019;18(1):142.
Wang Q, Guo X, Li L, Gao Z, Su X, Ji M, et al. N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 2020;11(10):911.
Du L, Li Y, Kang M, Feng M, Ren Y, Dai H, et al. USP48 is upregulated by Mettl14 to attenuate hepatocellular carcinoma via regulating SIRT6 stabilization. Cancer Res. 2021;81:3822–34.
Chen S, Yang C, Wang ZW, Hu JF, Pan JJ, Liao CY, et al. CLK1/SRSF5 pathway induces aberrant exon skip** of METTL14 and Cyclin L2 and promotes growth and metastasis of pancreatic cancer. J Hematol Oncol. 2021;14(1):60.
Wang F, Liao Y, Zhang M, Zhu Y, Wang W, Cai H, et al. N6-methyladenosine demethyltransferase FTO-mediated autophagy in malignant development of oral squamous cell carcinoma. Oncogene. 2021;40:3885–98.
Bian X, Shi D, **ng K, Zhou H, Lu L, Yu D, et al. AMD1 upregulates hepatocellular carcinoma cells stemness by FTO mediated mRNA demethylation. Clin Transl Med. 2021;11(3):e352.
Tao L, Mu X, Chen H, ** D, Zhang R, Zhao Y, et al. FTO modifies the m6A level of MALAT and promotes bladder cancer progression. Clin Transl Med. 2021;11(2):e310.
Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)a/MYC/CEBPA signaling. Cell. 2018;172(1–2):90–105 e123.
Dang Y, Xu J, Yang Y, Li C, Zhang Q, Zhou W, et al. Ling-gui-zhu-Gan decoction alleviates hepatic steatosis through SOCS2 modification by N6-methyladenosine. Biomed Pharmacother. 2020;127:109976.
Dang Y, Hu D, Xu J, Li C, Tang Y, Yang Z, et al. Comprehensive analysis of 5-hydroxymethylcytosine in zw10 kinetochore protein as a promising biomarker for screening and diagnosis of early colorectal cancer. Clin Transl Med. 2020;10(3):e125.
Dang Y, Xu J, Zhu M, Zhou W, Zhang L, Ji G. Gan-Jiang-Ling-Zhu decoction alleviates hepatic steatosis in rats by the miR-138-5p/CPT1B axis. Biomed Pharmacother. 2020;127:110127.
Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56(11):1585–9.
Mao X, Li P, Wang Y, Liang Z, Liu J, Li J, et al. CRB3 regulates contact inhibition by activating the hippo pathway in mammary epithelial cells. Cell Death Dis. 2017;8(1):e2546.
Fernando RN, Cotter L, Perrin-Tricaud C, Berthelot J, Bartolami S, Pereira JA, et al. Optimal myelin elongation relies on YAP activation by axonal growth and inhibition by Crb3/hippo pathway. Nat Commun. 2016;7:12186.
Burgers K, Moore C, Bednash L. Care of the Colorectal Cancer Survivor. Am Fam Physician. 2018;97(5):331–6.
Chen C, Yuan W, Zhou Q, Shao B, Guo Y, Wang W, et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics. 2021;11(9):4298–315.
Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH, Wang F, et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology. 2017;65(2):529–43.
Chen H, Gao S, Liu W, Wong CC, Wu J, Wu J, et al. RNA N(6)-Methyladenosine methyltransferase METTL3 facilitates colorectal Cancer by activating the m(6)A-GLUT1-mTORC1 Axis and is a therapeutic target. Gastroenterology. 2021;160(4):1284–300 e1216.
Chang YZ, Chai RC, Pang B, Chang X, An SY, Zhang KN, et al. METTL3 enhances the stability of MALAT1 with the assistance of HuR via m6A modification and activates NF-kappaB to promote the malignant progression of IDH-wildtype glioma. Cancer Lett. 2021;511:36–46.
Huang S, Luo S, Gong C, Liang L, **ao Y, Li M, et al. MTTL3 upregulates microRNA-1246 to promote occurrence and progression of NSCLC via targeting paternally expressed gene 3. Mol Ther Nucleic Acids. 2021;24:542–53.
Huo FC, Zhu ZM, Zhu WT, Du QY, Liang J, Mou J. METTL3-mediated m(6) a methylation of SPHK2 promotes gastric cancer progression by targeting KLF2. Oncogene. 2021;40(16):2968–81.
Li H, Zhang N, Jiao X, Wang C, Sun W, He Y, et al. Downregulation of microRNA-6125 promotes colorectal cancer growth through YTHDF2-dependent recognition of N6-methyladenosine-modified GSK3beta. Clin Transl Med. 2021;11(10):e602.
Karp CM, Tan TT, Mathew R, Nelson D, Mukherjee C, Degenhardt K, et al. Role of the polarity determinant crumbs in suppressing mammalian epithelial tumor progression. Cancer Res. 2008;68(11):4105–15.
Iioka H, Saito K, Kondo E. Crumbs3 regulates the expression of glycosphingolipids on the plasma membrane to promote colon cancer cell migration. Biochem Biophys Res Commun. 2019;519(2):287–93.
Li P, Feng C, Chen H, Jiang Y, Cao F, Liu J, et al. Elevated CRB3 expression suppresses breast cancer stemness by inhibiting beta-catenin signalling to restore tamoxifen sensitivity. J Cell Mol Med. 2018;22(7):3423–33.
Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. Crumbs3-mediated polarity directs airway epithelial cell fate through the hippo pathway effector yap. Dev Cell. 2015;34(3):283–96.
** L, Chen Y, Cheng D, He Z, Shi X, Du B, et al. YAP inhibits autophagy and promotes progression of colorectal cancer via upregulating Bcl-2 expression. Cell Death Dis. 2021;12(5):457.
Shen H, Huang C, Wu J, Li J, Hu T, Wang Z, et al. SCRIB promotes proliferation and metastasis by targeting hippo/YAP Signalling in colorectal Cancer. Front Cell Dev Biol. 2021;9:656359.
Tian C, Lang T, Qiu J, Han K, Zhou L, Min D, et al. SKP1 promotes YAP-mediated colorectal cancer stemness via suppressing RASSF1. Cancer Cell Int. 2020;20(1):579.
Sun Z, Zhang Q, Yuan W, Li X, Chen C, Guo Y, et al. MiR-103a-3p promotes tumour glycolysis in colorectal cancer via hippo/YAP1/HIF1A axis. J Exp Clin Cancer Res. 2020;39(1):250.
Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, et al. Small molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593:597–601.
Acknowledgments
We would like to thank KangChen Biotech (Shanghai, China) for its m6A epitranscriptomic microarray service and Shanghai Outdo Biotech for its tissue microarray service.
Funding
This work was supported by the National Natural Science Foundation of China (81804018 and 81620108030), the Shanghai Rising-Star Program (21QA1409000), and Shanghai Frontier Research Base of Disease and Syndrome Biology of Inflammatory cancer transformation (2021KJ03–12).
Author information
Authors and Affiliations
Contributions
GJ and YD conceived, designed, and supervised the study. YD, FL, and YX collected the samples. JP, FL, XX, and RX performed the all experiments; YD, FL, XX, LD, and MZ analyzed the data. YD and JP wrote the paper. AZ, WZ, and HX edited and revised the paper. All authors have reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Longhua Hospital (2019LCSY020), and informed consent was obtained from all participants.
Consent for publication
All authors have agreed to the publication of this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Expression of N6-methyladnosine methyltransferases and demethylases in both adenoma and colorectal carcinoma.
Additional file 2: Figure S2.
Validation of downstream targets after methyltransferase-like 3 knockdown.
Additional file 3: Figure S3.
The expression of YTH domain–containing family protein 2 was verified in adenoma and colorectal carcinoma tissues.
Additional file 4: Table S1.
Primer sequences of mRNAs in real-time PCR experiments. Table S2. Correlation between N6-methyladnosine (m6A) level and multiple clinical characteristics. Table S3. Cox regression analyses of m6A level in patients with CRC. Table S4. Correlation between methyltransferase-like 3 (METTL3) expression and multiple clinical characteristics. Table S5. Cox regression analyses of METTL3 expression in patients with CRC. Table S6. Primer sequences of MeRIP-qPCR and RIP.
Additional file 5.
mRNA methylation level in colorectal carcinoma vs. normal tissue.
Additional file 6.
mRNA quantity level in colorectal carcinoma vs. normal tissue.
Additional file 7: Figure S4.
All images of m6A immunofluorescence were showed.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pan, J., Liu, F., **ao, X. et al. METTL3 promotes colorectal carcinoma progression by regulating the m6A–CRB3–Hippo axis. J Exp Clin Cancer Res 41, 19 (2022). https://doi.org/10.1186/s13046-021-02227-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-021-02227-8