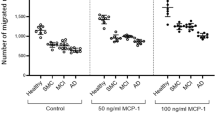
Abstract
Background
Neuroinflammation is a hallmark of neurodegenerative disease and a significant component of the pathology of Alzheimer’s disease (AD). Patients present with extensive microgliosis along with elevated pro-inflammatory signaling in the central nervous system and periphery. However, the role of peripheral myeloid cells in mediating and influencing AD pathogenesis remains unresolved.
Methods
Peripheral myeloid cells were isolated from peripheral blood of patients with prodromal AD (n = 44), mild AD dementia (n = 25), moderate/severe AD dementia (n = 28), and age-matched controls (n = 54). Patients were evaluated in the clinic for AD severity and categorized using Clinical Dementia Rating (CDR) scale resulting in separation of patients into prodromal AD (CDR0.5) and advancing forms of AD dementia (mild-CDR1 and moderate/severe-CDR2/3). Separation of peripheral myeloid cells into mature monocytes or immature MDSCs permitted the delineation of population changes from flow cytometric analysis, RNA phenotype analysis, and functional studies using T cell suppression assays and monocyte suppression assays.
Results
During stages of AD dementia (CDR1 and 2/3) peripheral myeloid cells increase their pro-inflammatory gene expression while at early stages of disease (prodromal AD—CDR0.5) pro-inflammatory gene expression is decreased. MDSCs are increased in prodromal AD compared with controls (16.81% vs 9.53%) and have markedly increased suppressive functions: 42.4% suppression of activated monocyte-produced IL-6 and 78.16% suppression of T cell proliferation. In AD dementia, MDSC populations are reduced with decreased suppression of monocyte IL-6 (5.22%) and T cell proliferation (37.61%); the reduced suppression coincides with increased pro-inflammatory signaling in AD dementia monocytes.
Conclusions
Peripheral monocyte gene expression is pro-inflammatory throughout the course of AD, except at the earliest, prodromal stages when pro-inflammatory gene expression is suppressed. This monocyte biphasic response is associated with increased numbers and suppressive functions of MDSCs during the early stages and decreased numbers and suppressive functions in later stages of disease. Prolonging the early protective suppression and reversing the later loss of suppressive activity may offer a novel therapeutic strategy.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is the most common neurodegenerative disease and is characterized by cognitive impairment, amyloid-β (Aβ) deposition, and neurofibrillary tangle formation [1,2,3,4,5]. Increasing evidence suggests that immune mechanisms contribute to the pathogenesis of AD including reactive microgliosis in postmortem samples, increased microglial activation marker, translocator protein (TSPO), binding on positron emission tomography (PET), [6] and increased pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, TNF, and IFN-γ in the cerebrospinal fluid and serum [7,8,9,10,11]. Thus, neuroinflammation is potentially a target for immunomodulatory therapies [12, 13].
Genome-wide association studies implicate immune system dysfunction, particularly in myeloid-derived cells, as immune-related genes coding for triggering receptor expressed on myeloid cells 2 (TREM2) and CD33 confer increased risk for AD susceptibility [14,15,16,17,18]. Current studies focus on microglia, resident myeloid cells of the brain, throughout AD pathogenesis, but recent literature in neurodegenerative diseases suggest extensive neuro-immune cross-talk between the brain and peripheral immune system [19,20,21,22]. This cross-talk may derive either directly or indirectly from peripheral immune cells in the presence of a compromised blood brain barrier (BBB) such as in neurodegenerative disease [23,24,25]. Additionally during inflammatory insult and microglial depletion, peripheral macrophage engraftment into the CNS was observed with these cells retaining a distinct and lasting transcriptional and functional identity [26]. Thus peripheral immune myeloid cells could modulate disease progression and outcomes in the CNS. The accessibility of these peripheral myeloid cells, and the lack of accessibility to CNS microglia, prompted a detailed examination of blood monocyte populations during the pathoprogression of AD.
Immature and mature monocytes, here-after denoted as “peripheral myeloid cells,” originate from hematopoietic stem cells and mature into peripheral monocytes with the capability of differentiating into macrophages once they enter tissue parenchyma [27,28,29,30,31]. Changes and shifts in peripheral myeloid populations are indicators of disease onset and progression for a multitude of diseases; the pro-inflammatory phenotypes have direct effects on their specific disease [32,33,34,35,36,37,38,39,40]. A detailed analysis of peripheral monocyte population and phenotype changes have not been documented thoroughly in AD progression.
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells that exhibit robust suppressive function on T cell proliferation and mature myeloid cell function which has made them a target of multiple immunomodulatory therapies [41,42,26]. Additionally, complex networks and signaling cascades involving additional cells outside our examination might assist in dictating inflammatory environments and cell phenotypes. Examination of these cells, both individually and in concert with each other, will help establish a more definitive immune environment during AD and through its course.
Conclusions
This study documents that peripheral monocytes are pro-inflammatory in advancing stages of AD but not in prodromal AD. The pro-inflammatory responses of monocytes from prodromal AD patients are suppressed while advancing AD patients monocytes lose this suppression, and become activated and pro-inflammatory. Numbers and suppressive functions of MDSCs are increased in prodromal AD and decreased in patients with advancing AD and correlate with pro-inflammatory expression of AD monocytes. MDSCs also suppress Tresp which can readily enter the CNS, and loss of T effector suppression can significantly enhance inflammatory disease pathology. These findings provide a novel inflammatory paradigm that may have confounded early therapeutic interventions and provides a new basis for how future studies and treatments should be designed. Additionally, we have documented the significant impact of AD MDSCs on immune cell subsets. Understanding the role of early enhanced immunosuppression in prodromal AD and the subsequent dysfunction of this process in AD dementia may lead to novel therapeutic strategies.
References
Brion JP. Neurofibrillary tangles and Alzheimer's disease. Eur Neurol. 1998;40:130–40.
Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–5.
Larson EB, Kukull WA, Katzman RL. Cognitive impairment: dementia and Alzheimer's disease. Annu Rev Public Health. 1992;13:431–49.
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6.
Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr71.
Kreisl WC, Lyoo CH, Liow JS, Wei M, Snow J, Page E, Jenko KJ, Morse CL, Zoghbi SS, Pike VW, et al. (11)C-PBR28 binding to translocator protein increases with progression of Alzheimer's disease. Neurobiol Aging. 2016;44:53–61.
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421.
Cuello AC. Early and late CNS inflammation in Alzheimer's disease: two extremes of a continuum? Trends Pharmacol Sci. 2017;38:956–66.
Hopperton KE, Mohammad D, Trepanier MO, Giuliano V, Bazinet RP. Markers of microglia in post-mortem brain samples from patients with Alzheimer's disease: a systematic review. Mol Psychiatry. 2018;23:177–98.
Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2:a006346.
Zimmer ER, Leuzy A, Benedet AL, Breitner J, Gauthier S, Rosa-Neto P. Tracking neuroinflammation in Alzheimer's disease: the role of positron emission tomography imaging. J Neuroinflammation. 2014;11:120.
Schwab C, Klegeris A, McGeer PL. Inflammation in transgenic mouse models of neurodegenerative disorders. Biochim Biophys Acta. 2010;1802:889–902.
Wilcock DM, Zhao Q, Morgan D, Gordon MN, Everhart A, Wilson JG, Lee JE, Colton CA. Diverse inflammatory responses in transgenic mouse models of Alzheimer's disease and the effect of immunotherapy on these responses. ASN Neuro. 2011;3:249–58.
Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A, Rosenkrantz LL, Imboywa S, Lee M, Von Korff A, et al. CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 2013;16:848–50.
Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–43.
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368:117–27.
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368:107–16.
Malik M, Simpson JF, Parikh I, Wilfred BR, Fardo DW, Nelson PT, Estus S. CD33 Alzheimer's risk-altering polymorphism, CD33 expression, and exon 2 splicing. J Neurosci. 2013;33:13320–5.
Goldeck D, Witkowski JM, Fulop T, Pawelec G. Peripheral immune signatures in Alzheimer disease. Curr Alzheimer Res. 2016;13:739–49.
Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–71.
Le Page A, Dupuis G, Frost EH, Larbi A, Pawelec G, Witkowski JM, Fulop T. Role of the peripheral innate immune system in the development of Alzheimer's disease. Exp Gerontol. 2018;107:59–66.
Varvel NH, Neher JJ, Bosch A, Wang W, Ransohoff RM, Miller RJ, Dingledine R. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc Natl Acad Sci U S A. 2016;113:E5665–74.
Carvey PM, Hendey B, Monahan AJ. The blood-brain barrier in neurodegenerative disease: a rhetorical perspective. J Neurochem. 2009;111:291–314.
de Vries HE, Kooij G, Frenkel D, Georgopoulos S, Monsonego A, Janigro D. Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia. 2012;53(Suppl 6):45–52.
Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19:121–30.
Cronk JC, Filiano AJ, Louveau A, Marin I, Marsh R, Ji E, Goldman DH, Smirnov I, Geraci N, Acton S, et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J Exp Med. 2018;215:1627–47.
Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front Cell Neurosci. 2013;7:45.
London A, Cohen M, Schwartz M. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front Cell Neurosci. 2013;7:34.
Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 2013;35:601–12.
Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1. https://doi.org/10.1186/2050-7771-2-1.
Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80.
Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12:e0176460.
Gawdat K, Legere S, Wong C, Myers T, Marshall JS, Hassan A, Brunt KR, Kienesberger PC, Pulinilkunnil T, Legare JF. Changes in circulating monocyte subsets (CD16 expression) and neutrophil-to-lymphocyte ratio observed in patients undergoing cardiac surgery. Front Cardiovasc Med. 2017;4:12.
Kim WK, Sun Y, Do H, Autissier P, Halpern EF, Piatak M Jr, Lifson JD, Burdo TH, McGrath MS, Williams K. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J Leukoc Biol. 2010;87:557–67.
Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-classical monocytes display inflammatory features: validation in Sepsis and systemic lupus erythematous. Sci Rep. 2015;5:13886.
Ong SM, Hadadi E, Dang TM, Yeap WH, Tan CT, Ng TP, Larbi A, Wong SC. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 2018;9:266.
Stansfield BK, Ingram DA. Clinical significance of monocyte heterogeneity. Clin Transl Med. 2015;4:5.
Wildgruber M, Aschenbrenner T, Wendorff H, Czubba M, Glinzer A, Haller B, Schiemann M, Zimmermann A, Berger H, Eckstein HH, et al. The "intermediate" CD14(++)CD16(+) monocyte subset increases in severe peripheral artery disease in humans. Sci Rep. 2016;6:39483.
Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L, Brenner D, Martin-Villalba A, Hengerer B, Kassubek J, et al. Inflammatory dysregulation of blood monocytes in Parkinson's disease patients. Acta Neuropathol. 2014;128:651–63.
Zhao W, Beers DR, Hooten KG, Sieglaff DH, Zhang A, Kalyana-Sundaram S, Traini CM, Halsey WS, Hughes AM, Sathe GM, et al. Characterization of gene expression phenotype in amyotrophic lateral sclerosis monocytes. JAMA Neurol. 2017;74:677–85.
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150.
Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–7.
Kong YY, Fuchsberger M, **ang SD, Apostolopoulos V, Plebanski M. Myeloid derived suppressor cells and their role in diseases. Curr Med Chem. 2013;20:1437–44.
Tamadaho RSE, Hoerauf A, Layland LE. Immunomodulatory effects of myeloid-derived suppressor cells in diseases: role in cancer and infections. Immunobiology. 2018;223:432–42.
Salminen A, Kaarniranta K, Kauppinen A. The potential importance of myeloid-derived suppressor cells (MDSCs) in the pathogenesis of Alzheimer's disease. Cell Mol Life Sci. 2018;75:3099. https://doi.org/10.1007/s00018-018-2844-6.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–9.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9.
Yanagimachi MD, Niwa A, Tanaka T, Honda-Ozaki F, Nishimoto S, Murata Y, Yasumi T, Ito J, Tomida S, Oshima K, et al. Robust and highly-efficient differentiation of functional monocytic cells from human pluripotent stem cells under serum- and feeder cell-free conditions. PLoS One. 2013;8:e59243.
Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–97.
Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–12.
Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–49.
Meyer PF, Savard M, Poirier J, Labonte A, Rosa-Neto P, Weitz TM, Town T, Breitner J. Alzheimer's disease neuroimaging I, group P-AR: bi-directional Association of Cerebrospinal Fluid Immune Markers with stage of Alzheimer's disease pathogenesis. J Alzheimers Dis. 2018;63:577–90.
Fan Z, Brooks DJ, Okello A, Edison P. An early and late peak in microglial activation in Alzheimer's disease trajectory. Brain. 2017;140:792–803.
Feng Y, Li L, Sun XH. Monocytes and Alzheimer's disease. Neurosci Bull. 2011;27:115–22.
Martin E, Boucher C, Fontaine B, Delarasse C. Distinct inflammatory phenotypes of microglia and monocyte-derived macrophages in Alzheimer's disease models: effects of aging and amyloid pathology. Aging Cell. 2017;16:27–38.
Saresella M, Marventano I, Calabrese E, Piancone F, Rainone V, Gatti A, Alberoni M, Nemni R, Clerici M. A complex proinflammatory role for peripheral monocytes in Alzheimer's disease. J Alzheimers Dis. 2014;38:403–13.
Theriault P, ElAli A, Rivest S. The dynamics of monocytes and microglia in Alzheimer's disease. Alzheimers Res Ther. 2015;7:41.
Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31.
Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–75.
Le Page A, Garneau H, Dupuis G, Frost EH, Larbi A, Witkowski JM, Pawelec G, Fulop T. Differential phenotypes of myeloid-derived suppressor and T regulatory cells and cytokine levels in amnestic mild cognitive impairment subjects compared to mild Alzheimer diseased patients. Front Immunol. 2017;8:783.
Wisniewski HM, Kozlowski PB. Evidence for blood-brain barrier changes in senile dementia of the Alzheimer type (SDAT). Ann N Y Acad Sci. 1982;396:119–29.
Wisniewski HM, Vorbrodt AW, Wegiel J. Amyloid angiopathy and blood-brain barrier changes in Alzheimer's disease. Ann N Y Acad Sci. 1997;826:161–72.
Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging. 2007;28:977–86.
Alafuzoff I, Adolfsson R, Bucht G, Winblad B. Albumin and immunoglobulin in plasma and cerebrospinal fluid, and blood-cerebrospinal fluid barrier function in patients with dementia of Alzheimer type and multi-infarct dementia. J Neurol Sci. 1983;60:465–72.
Blennow K, Wallin A, Fredman P, Karlsson I, Gottfries CG, Svennerholm L. Blood-brain barrier disturbance in patients with Alzheimer's disease is related to vascular factors. Acta Neurol Scand. 1990;81:323–6.
Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries CG, Blennow K. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer's disease and vascular dementia. Neurology. 1998;50:966–71.
Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302.
Yamazaki Y, Kanekiyo T. Blood-brain barrier dysfunction and the pathogenesis of Alzheimer's disease. Int J Mol Sci. 2017;18.
Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20:136–44.
Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: D-day for neurodegenerative disease? J NeuroImmune Pharmacol. 2009;4:462–75.
El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–8.
Harms AS, Delic V, Thome AD, Bryant N, Liu Z, Chandra S, Jurkuvenaite A, West AB. Alpha-Synuclein fibrils recruit peripheral immune cells in the rat brain prior to neurodegeneration. Acta Neuropathol Commun. 2017;5:85.
Harms AS, Thome AD, Yan Z, Schonhoff AM, Williams GP, Li X, Liu Y, Qin H, Benveniste EN, Standaert DG. Peripheral monocyte entry is required for alpha-Synuclein induced inflammation and neurodegeneration in a model of Parkinson disease. Exp Neurol. 2018;300:179–87.
Beury DW, Parker KH, Nyandjo M, Sinha P, Carter KA, Ostrand-Rosenberg S. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. J Leukoc Biol. 2014;96:1109–18.
Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–54.
Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–91.
Acknowledgements
We would like to thank the Stanley H. Appel Department of Neurology, the Nantz National Alzheimer Center, and Houston Methodist Hospital for their contributions and patient organization/recruitment. Individually, we acknowledge Douglas Casey, **ghong Wang, and Neha Pal for their time and efforts in the completion of this project. We also thank patients and their families for their participation and involvement in the study.
Funding
This study was funded by the Ting Tsung and Wei Fong Chao Foundation in Houston, TX.
Availability of data and materials
Materials and/or datasets used/generated are included in the manuscript or available upon reasonable request.
Author information
Authors and Affiliations
Contributions
ADT and SHA conceived and designed research; ADT, AF, JRT, WZ, and SW performed experiments; ADT, DRB, JRT, WZ, and SHA analyzed and interpreted the data. AF, BP, and JCM recruited, organized clinical operations for sample collection, and rated/categorized patients; ADT wrote manuscript with input from all authors. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Houston Methodist IRB and all participants signed informed consent.
Consent for publication
This manuscript has been read and approved by all authors, it has not been previously published, and is not under simultaneous consideration by another journal. Authors give consent for publication in Molecular Neurodegeneration.
Competing interests
The authors of this manuscript declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Cell Populations. (TIF 58 kb)
Additional file 2:
Figure S2. Flow cytometry gating schemes for monocyte and MDSC populations. (TIF 539 kb)
Additional file 3:
Figure S9. Nanostring dataset for RNA analysis of CDR rated patients and age-matched controls. (XLSX 65 kb)
Additional file 4:
Figure S4. iPSC-derived M1 and MDSC co-culture paradigm. (TIF 231 kb)
Additional file 5:
Figure S3. (a) Correlation data of inflammatory RNA expression and subject age from peripheral myeloid cells. Data represent analyses of RNA expression with controls only, patients with varying levels of AD, and all groups combined (Control n = 20, AD n = 38). (b) Analyses of gender contributions to pro-inflammatory RNA expression among controls, CDR0.5, CDR1, and CDR2/3 (Control n = 10/10 M/F, CDR0.5 n = 6/14 M/F, CDR1 n = 5/3 M/F, CDR2/3 n = 4/6 M/F). Graphs show average ± SEM with statistics run using two-way ANOVA with Sidak’s multiple comparisons test. No statistical difference observed after age and gender data stratification unless signified by *p < 0.05, **p < 0.01, or ***p < 0.001. (TIF 156 kb)
Additional file 6:
Figure S6. (a) Correlation data between age and protein expression of HLADR and CD33 analyzed via flow cytometry (Control n = 30, AD n = 57). (b) Analyses of gender contributions to HLADR and CD33 expression on mature myeloid cells isolated from controls, CDR0.5, CDR1, and CDR2/3 (Control n = 14/16 M/F, CDR0.5 n = 13/14 M/F, CDR1 n = 8/10 M/F, CDR2/3 n = 3/10 M/F). Graphs show average ± SEM with statistics run using two-way ANOVA with Sidak’s multiple comparisons test. No statistical difference observed after age and gender data stratification unless signified by *p < 0.05, **p < 0.01, or ***p < 0.001. (TIF 194 kb)
Additional file 7:
Figure S5. (a) Correlation data between age and monocyte population changes. Analyses performed examined the ages of controls, varying levels of AD, and combined groups for correlations in changes in classical monocytes, intermediate monocytes, non-classical monocytes, and MDSCs (Control n = 35, AD n = 66). (b) Analyses of gender contributions to monocyte population changes among controls, CDR0.5, CDR1, and CDR2/3 (Control n = 20/15 M/F, CDR0.5 n = 15/16 M/F, CDR1 n = 8/10 M/F, CDR2/3 n = 5/12 M/F). Graphs show average ± SEM with statistics run using two-way ANOVA with Sidak’s multiple comparisons test. No statistical difference observed after age and gender data stratification unless signified by *p < 0.05, **p < 0.01, or ***p < 0.001. (TIF 246 kb)
Additional file 8:
Figure S7. (a) Analysis of gender contribution to MDSC suppressive function on pro-inflammatory M1 cells (Control n = 6/4 M/F, CDR0.5 n = 5/6 M/F, CDR1 n = 4/6 M/F, CDR2/3 n = 3/7 M/F). Graph shows average ± SEM with statistics run using two-way ANOVA with Sidak’s multiple comparisons test. No statistical difference observed after gender data stratification unless signified by *p < 0.05, **p < 0.01, or ***p < 0.001. (TIF 25 kb)
Additional file 9:
Figure S8. (a) Correlation plot graphing T resp. proliferation suppression and myeloid IL-6 transcript suppression at 1:1 ratio of responding cells to MDSCs (R = .7288 p = 0.004). (b) IL-6 control experiment whereby MDSCs from controls (n = 6) and AD patients from various stages (n = 12) do not express IL-6 transcript when cultured alone in LPS/IFNγ treatments. Corroboration with no IL-6 protein in the MDSC only treated media when analyzed via ELISA (data not shown). (TIF 29 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Thome, A.D., Faridar, A., Beers, D.R. et al. Functional alterations of myeloid cells during the course of Alzheimer’s disease. Mol Neurodegeneration 13, 61 (2018). https://doi.org/10.1186/s13024-018-0293-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13024-018-0293-1