Abstract
Porcine reproductive and respiratory syndrome (PRRS) is endemic worldwide, seriously affecting the development of the pig industry, but vaccines have limited protective effects against PRRSV transmission. The aim of this study was to identify potential anti-PRRSV drugs. We examined the cytotoxicity of seven compounds formulated based on the mass ratio of glycyrrhizic acid to matrine and calculated their inhibition rates against PRRSV in vitro. The results showed that the seven compounds all had direct killing and therapeutic effects on PRRSV, and the compounds inhibited PRRSV replication in a time- and dose-dependent manner. The compound with the strongest anti-PRRSV effect was selected for subsequent in vivo experiments. Pigs were divided into a control group and a medication group for the in vivo evaluation. The results showed that pigs treated with the 4:1 compound had 100% morbidity after PRRSV challenge, and the mortality rate reached 75% on the 8th day of the virus challenge. These results suggest that this compound has no practical anti-PRRSV effect in vivo and can actually accelerate the death of infected pigs. Next, we further analyzed the pigs that exhibited semiprotective effects following vaccination with the compound to determine whether the compound can synergize with the vaccine in vivo. The results indicated that pigs treated with the compound had higher mortality rates and more severe clinical reactions after PRRSV infection (p < 0.05). The levels of proinflammatory cytokines (IL-6, IL-8, IL-1β, IFN-γ, and TNF-α) were significantly greater in the compound-treated pigs than in the positive control-treated pigs (p < 0.05), and there was no synergistic enhancement with the live attenuated PRRSV vaccine (p < 0.05). The compound enhanced the inflammatory response, prompted the body to produce excessive levels of inflammatory cytokines and caused body damage, preventing a therapeutic effect. In conclusion, the present study revealed that the in vitro effectiveness of these agents does not indicate that they are effective in vivo or useful for develo** anti-PRRSV drugs. Our findings also showed that, to identify effective anti-PRRSV drugs, comprehensive drug screening is needed, for compounds with solid anti-inflammatory effects both in vitro and in vivo. Our study may aid in the development of new anti-PRRSV drugs.
Similar content being viewed by others
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is caused by porcine reproductive and respiratory syndrome virus (PRRSV) and is one of the most common infectious diseases affecting the pig industry worldwide [1]. The clinical manifestations of PRRS are reproductive dysfunction in sows, respiratory diseases in growing pigs, and slow growth and high mortality rates in weaned pigs, collectively causing enormous economic losses in pig production [2, 3]. PRRSV is a small enveloped RNA virus with a linear, single-stranded, sense genome and belongs to the Arteriviridae family of the order Nidovirales [4]. PRRSV is internalized into host cells through interactions between PRRSV proteins and cellular receptors. Upon viral invasion of the cell, the host’s antiviral immune system is rapidly activated to inhibit viral replication. To remain host-adapted, some viruses have evolved a variety of sophisticated strategies to manipulate the host machinery and circumvent the host’s antiviral response [5]. Currently, vaccination strategies, including those in which live-attenuated vaccines, modified live viruses vaccines, DNA vaccines and immune-adjuvanted vaccines are used are the most cost-effective means to prevent PRRS [6,7,8]. However, due to the diversity of epidemic strains, these vaccines are incapable of producing sustainable disease prevention and control, and safer and more efficient vaccines have not been developed that have advanced to the clinical stage [9, 10]. Research on new vaccines and antiviral drugs is needed to identify effective methods for the prevention and control of PRRS, which continues to be a high priority in the global pig industry [11].
Traditional Chinese medicines (TCMs) contain very complex chemical components and have various clinical applications. Many natural products and their derivatives have been confirmed to have natural antiviral effects [12]. TCMs are rich in resources, have few drug residues, and can enhance the immunity. With the development of advanced molecular and analytical techniques, more antiviral and immunomodulatory effects of TCMs and their derivatives, such as glycosides, terpenoids, isoflavones, alkaloids, and asiatic acid, have been discovered and clarified [11, 13,14,15]. Currently, studies have confirmed that several bioactive compounds extracted from TCMs have potent antiviral activities against PRRSV in vitro based on different antiviral strategies [16, 17], including those that prevent the viruses from attaching to putative receptors or blocking their entrance into cells in vitro by interfering with the process that leads to the uncoating of the viral genome. (–)-Epigallocatechin-3-gallate (EGCG), a natural bioactive compound isolated from green tea [18], has multiple functions, including inhibiting PRRSV replication in MARC-145 cells in vitro [11]. Curcumin is the most abundant bioactive compound in the rhizomes of Curcuma longa, and it has been reported to inhibit the entry of PRRSV into MARC-145 and PAM cells by interfering with all postinternalization stages of PRRSV [19].
The components of TCMs that exert antiviral effects include both individual components and groups of components [20,21,Full size image
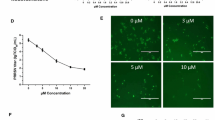
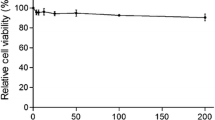
Ribavirin is a well-known viral RNA polymerase inhibitor that has broad-spectrum antiviral activity against infections caused by RNA viruses, including the hepatitis C virus, respiratory syncytial virus (RSV), poliovirus, and hepatitis A and B type influenza viruses (IAV and IBV). Therefore, we used 140 mM ribavirin as a positive antiviral drug control. As shown in Table 1, although the therapeutic index of ribavirin was greater than that of the other compound prescriptions, with a TI value of 31.78, its inhibition rate was lower than that of glycyrrhizic acid and matrine at a mass ratio of 4:1 and a concentration of 0.25 mg/mL. Overall, we determined that glycyrrhizic acid: matrine was the optimal compound at a ratio of 4:1 by mass and this ratio was used to continue exploring the anti-PRRSV effect of the drug.
Next, we analyzed the effects of drugs at different concentrations and at different time points regarding the proliferation of PRRSV. The results are shown in Fig. 3. Between 2 and 12 h of viral infection, the drugs at all concentrations had significant inhibitory effects on the proliferation of PRRSV (p < 0.05). The antiviral effect of the drugs was greatest when the duration of infection was 2 h, but as the duration of viral infection increased, the effect of the drugs on the replication of the viruses decreased. When the viruses infected the cells for more than 24 h, the drugs had the greatest effects on PRRSV within the safe concentration range. There was no significant inhibitory effect on proliferation (p > 0.05).
MARC-145 cells were treated with glycyrrhizic acid and matrine at a mass ratio of 4:1, and the inhibition activity was determined at different concentrations and different time points. The data are from three independent experiments. The error bars denote standard errors of the means.
When the drug was used at 250 µg/mL, the TCID50 decreased by 1.13-fold compared with that of the virus control, and the effect of the drug on the PRRSV TCID50 was positively correlated with its concentration. When the drug concentration was 500 µg/mL, the TCID50 of the virus was 0 (Table 2).
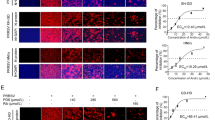
We detected the activation of PRRSV in the cell control, virus control, 0.25 mg/mL compound group and 0.125 mg/mL ribavirin groups of MARC-145 cells at 72 h post infection (hpi). As shown in Fig. 4A, the nuclei were stained blue after DAPI application. The states of the cell nuclei in the control group and the compound drug group were similar; the nuclei were round, and the excitation colors after staining were uniform. In the virus control group and the ribavirin group, the nuclei were shrunken and had a grainy appearance. As shown in Fig. 4B, FITC-labeled N protein was stained green. The cells in the control group had no fluorescence, those in the ribavirin control group had fluorescence, those in the compound drug group had weak fluorescence, and those in the virus control group had strong fluorescence. Figure 4C shows the results of the fluorescence fusion of cells and nuclei in each group of cells. These results suggest that the compound drugs matrine and glycyrrhizinate have anti-PRRSV effects and that the effect of the compound drug is slightly better than that of ribavirin.
Glycyrrhizic acid and matrine (4:1) inhibited the PRRSV-N protein expression. A. Nuclear staining; B. PRRSV-N protein staining; C. nuclear and PRRSV-N protein staining. MARC-145 cells were treated with G: M (4:1) for 72 h. Ribavirin was used as a positive control. The cells were examined by laser confocal microscopy. Bars, 100 μm
The compound drug failed to exhibit a practical anti-PRRSV effect in vivo
To examine the therapeutic effect of matrine and glycyrrhizinate compounds in vivo, we randomly divided 8 pigs into 2 groups (challenge group and treatment group). The detailed grou** and challenge information are shown in Fig. 5A. Over 21 days after the completion of infection and drug treatment, we observed and evaluated the clinical symptoms of the pigs on a daily basis; the morbidity and mortality of the pigs are shown in Fig. 5A. After challenge with PRRSV, the pigs in each group developed typical PRRS symptoms, with an incidence rate of 100%. The typical clinical symptoms of PRRS, such as decreased appetite, poor breathing, red and swollen eyelids, and depression, appeared in the control group and the medication group 3 d and 4 d after challenge, respectively.
There was no significant difference in body temperature between the control group and the medication group after challenge (p > 0.05). As shown in Fig. 5B, the temperature of the control group and the medication group started to rise (above 40 °C) on the 1st day of challenge, peaked on the 5th day of the challenge, and then returned to the average temperature on the 12th day of the challenge. On the 7th day of the challenge, the temperature of the challenge group was significantly higher than that of the treatment group (p < 0.05).
As shown in Table 3, within 21 days of the challenge, the body weight of the challenge group showed a negative increase, and the total weight gain of the medication group was 0.11 kg, which was not a significant difference (p > 0.05).
As shown in Fig. 5C, except for the significant difference on Day 4 (p < 0.05) (the antibody levels were all negative at this time), the PRRSV N antibody levels between the control group and the medication group were not significantly different (p > 0.05). The antibody levels were basically the same for both groups.
As shown in Fig. 5A, there were differences in mortality between the control group and the medication group. In the control group, one pig died on Day 11, and the remaining pigs died on Day 15. In contrast, in the medication group, one pig died on the Day 4, and two died on Day 8. Based on the clinical symptoms, the medication group experienced more acute death after treatment, with the mortality rate reaching 75% after the 8th day of challenge; no deaths occurred in the control group.
Overall, the results showed that the compound drug failed to exhibit a practical anti-PRRSV effect in vivo; on the contrary, it accelerated the death rate of experimental pigs infected with PRRSV.
Clinical symptoms in pigs from the in vivo experiments. (A) Grou**, morbidity and death rates obtained from the in vivo experiment; (B) Temperature of the pigs; (C) Changes in the antibody against the PRRSV N protein in the pigs. The data are from three independent experiments. The error bars denote standard errors of the means
The compound drug does not synergistically enhance the anti-PRRSV effect of the vaccine
There may be various reasons for the inability of the compound drug to exert an anti-PRRSV effect in vivo. We wondered whether the therapeutic effect was insufficient due to the high viral load in the body after challenge, so we used half the usual dose of the live PRRSV vaccine for immunization. In pigs, we observed whether the compound drug can exert its effect against PRRSV while providing a semiprotective effect on the animals or whether it can synergize with the vaccine virus to exert a better protective effect in vivo. We randomly divided 20 pigs into 4 groups. The detailed grou** and challenge information is shown in Fig. 6A. After PRRSV challenge, one pig in the immunized group experienced clinical symptoms on the 3rd day, whereas the other four pigs experienced no clinical symptoms; the incidence rate was 20%, and no deaths occurred. In contrast, the 5 pigs in the nonimmunized drug group (the group that was not immunized or directly challenged) all exhibited the disease symptoms on Day 3 after challenge, and the incidence rate was 100%. Death began to occur on Day 8 after challenge. The nonimmunized control group also had clinical symptoms on Day 3 after challenge, with an incidence rate of 100%. Death began to occur on the 10th day after challenge, and 3 animals died, with the mortality rate reaching 60%.
Determination of the synergistic immune effect of the compound drug vaccine. (A) Experimental grou**s: immune drug group (IM), immunized control group (IMC), nonimmunized drug group (NIM), and nonimmunized control group (NIMC); (B) Antibody level of PRRSV N-protein in pigs; (C) Levels of IFN-α; (D) Levels of IL-1β; (E) Levels of IL-6; (F) Levels of TNF-α; (G) Levels of IL-8
The S/P ratios of antibodies against the PRRSV N protein after 3 d of challenge are shown in Fig. 6B. The nonimmunized group did not produce antibodies after 3 d or 5 d of challenge, and the difference between the immunized and nonimmunized groups was significant (p < 0.05). The difference between the immunized control and control groups was not significant (p > 0.05). Antibodies were detected in the nonimmunized group at 7 d of challenge. After 9 d of challenge, the postinfection antibody levels of the pigs in each group were consistent, and there was no significant difference between them (p > 0.05).
As shown in Fig. 6C-G, there were no significant differences in the cytokine levels of the nonimmunized groups during the immunization period, whereas the levels of the inflammatory cytokines IL-6, IL-8, IFN-α, and TNF-α in the immunization group were significantly different at 14d after immunization. After challenge, the cytokine levels in the nonimmunized drug group were significantly greater than those in the nonimmunized control group at multiple time points (p < 0.05); the cytokine levels in the nonimmunized group were greater after challenge than those in the immunized group at all time points. The results showed that matrine and glycyrrhizic acid enhanced the inflammatory response of pigs infected with the virulent PRRSV strain and prompted the body to produce excessive inflammatory cytokines that caused body damage, preventing a therapeutic effect from being produced. Pigs did not exhibit synergistic enhancement.