Abstract
Background
Despite centuries of research, debate remains on the scaling of metabolic rate to mass especially for intraspecific cases. The high variation of body mass within brown bears presents a unique opportunity to study the intraspecific effects of body mass on physiological variables. The amplitude of metabolic rate reduction in hibernators is dependent on body mass of the species. Small hibernators have high metabolic rates when euthermic but experience a drastic decrease in body temperature during torpor, which is necessary to reach a very low metabolic rate. Conversely, large hibernators, such as the brown bear (Ursus arctos), show a moderate decrease in temperature during hibernation, thought to be related to the bear’s large size. We studied body mass, abdominal body temperature, heart rate, and accelerometer-derived activity from 63 free-ranging brown bears (1–15 years old, 15–233 kg). We tested for relationships between body mass and body temperature, heart rate, and hibernation duration.
Results
The smallest individuals maintained lower body temperatures during hibernation, hibernated longer, and ended hibernation later than large bears. Unlike body temperature, winter heart rates were not associated with body mass. In summer, the opposite pattern was found, with smaller individuals having higher body temperature and daytime heart rates. Body mass was associated with body temperature in the winter hypometabolic state, even in a large hibernating mammal. Smaller bears, which are known to have higher thermal conductance, reached lower body temperatures during hibernation. During summer, smaller bears had higher body temperatures and daytime heart rates, a phenomenon not previously documented within a single mammalian species.
Conclusion
We conclude that the smallest bears hibernated more deeply and longer than large bears, likely from a combined effect of basic thermodynamics, the higher need for energy savings, and a lower cost of warming up a smaller body.
Similar content being viewed by others
Background
The relationship between body mass and body size between species has been described and studied for over a century [1]. The influence of body size on body temperature (Tb) and metabolic rate is well established across a variety of classes of vertebrates, including birds, amphibians, and reptiles [2]. When plotted against body mass, the metabolic rates across species, especially for mammals and birds, follow a logarithmic curve [3]. Clarke and Rothery 2008 reviewed data from 596 mammalian species and concluded that body mass, body temperature and metabolic rate are in a complex relationship, mediated through ecology [4]. Morrison and Ryser [5] clearly illustrated the effect of body size on Tb across a range of mammalian species from 0.001 to 100,000 kg. However, more recent studies illustrate the difficulty of using one model to predict metabolic rate across all mammalian species [6]. Different scaling exponents, ranging from 0.35 to 0.97, have been reported, for example, for the brown rat (Rattus norvegicus) [7]. A meta-review by Glazier 2005 concluded that metabolic scaling is complex and results from adaptations to physiochemical and ecological constraints [8]. Interspecific metabolic scaling in particular differs in regard to temperature regulation, body-size range and activity level. Although minimum Tb during hibernation increases with increasing body mass [9], no such relationship has been found for mammals during euthermia [10].
In many animal species, metabolic rate is tightly connected to heart rate (HR) and can be calibrated to metabolic rate with specific validation. Without specific validation, HR can only be used as a qualitative proxy for metabolic rate. The correlation between HR and body mass has been studied in several mammalian species with contrasting results. For example, no difference was found in comparing HR across different sizes of dogs [11, 12], although a study on horses and ponies found body weight to be a strong predictor of HR [13] and a recent study on Canadian lynx (Lynx canadensis) found higher HR in smaller individuals compared to larger ones [14]. Whereas in humans, age seems to be a strong predictor for intrinsic heart rate, declining with increasing age [15].
In the literature on mammalian hibernation the question of how mammals of different sizes, ranging from bats to bears, can hibernate and how an animal’s body size affects its status as a hibernator, has been a topic of interest for many years [16, 17]. Most studies on small mammals, which have regular arousals during hibernation (accounting for 72% of the total energy used in marmots during winter [18]), have focused on the effects of pre-hibernation body condition and energy (fat) reserves on subsequent winter hibernation. An interspecific comparison found no relationship between body size and length of torpor bouts across 76 species of hibernators [9]. It is also well accepted that body size determines the degree of reduction in metabolic rate between euthermic and torpid periods; with small hibernators having a higher metabolic rate when euthermic and demonstrating a drastic decrease in metabolic rates to reach low Tb in torpor [18]. Some studies have concluded that fatter individuals, i.e. with greater energy reserves, have a higher mean minimum body temperature and arouse more often, and for longer periods, than leaner animals during hibernation [19, 20]. This is likely to reduce the associated somatic costs of torpor (such as oxidative stress, reduced immunocompetence and neuronal damage[21]), indicating intraspecific variation of energetics during hibernation. In contrast, other studies [9, 22,23,24,25] have not found evidence of this.
In large hibernating species, such as brown bears (Ursus arctos), body mass and body condition (an index of body fat storage) are not related[26]. Body mass has instead been found to correlate with age in brown bears [27, 28]. Few hibernators span such a wide range of body mass as the brown bear, with spring body mass in Scandinavian brown bears ranging from 8 to 44 kg at one year of age [29] to 62–241 kg as adults [30]. This is a life-history trait considerably different from small hibernators, such as the Syrian golden hamster (Mesocricetus auratus) at 102–149 g [31], or the Alpine woodchuck (Marmota monax), which ranges from 2 to 5 kg from juvenile to adult [32]. The brown bear, with a range of body masses (reflecting body sizes) differing more than 30 times, is therefore deserving of special attention, providing the unique opportunity to study intraspecific variation of hibernation status in relation to body size.
The metabolic rate reduction during hibernation in brown bears, is likely similar to the 75% reduction reported in American black bears [33], which is in contrast to a 95% decrease on average in some small (< 5 kg) hibernators [34]. Based on the reviewed literature, the lower magnitude of temperature fluctuations during hibernation in bears, compared to other hibernators, is thought to be related to the bears’ large body size [18, 35, 36]. Body mass affects thermodynamics and one study in American black bears (Ursus americanus) found that larger bears had lower thermal conductance. Although smaller black bears had higher total body conductance, their lower critical temperatures during hibernation was not significantly higher than that of larger bears [37]. The hibernation-optimization hypothesis [38] suggests that hibernators that can afford to spend less time in torpor, and more time at euthermic Tb through arousals, reduce the negative effects of rewarming from torpor, including oxidative stress, reduced immunocompetence, and neuronal damage [21]. In this regard, even though bears do not show arousals, larger bears with more fat reserves would be expected to exhibit a shorter hibernation period, as has been previously shown for male brown bears [39], which are generally larger than female bears.
Overall, the large amount of data supporting the well-described interspecific effect of body size on metabolic rates and Tb in homeothermic endotherms vs heterothermic endotherms, contrast with the few studies available presenting intraspecific data, in particular for species in which body sizes changes dramatically over their lifespan, such as the brown bear. One study on American black bears (n = 12, body mass 35.5–116.5 kg) in captivity found that smaller bears had higher mass specific metabolic rates during hibernation[37]. So far, the question of whether intraspecific differences in body size are associated with different hibernating and energy saving patterns is understudied.
In this study, we investigated this question using a unique dataset of 63 free-ranging brown bears aged 1–15 years old, having a body mass range from 23 to 233 kg. We tested for relationships between body mass, body temperature and HR in winter and summer, in addition to hibernation duration and timing of emergence from hibernation.
Materials and methods
Study area
The study from southcentral Sweden (61°N, 15°E) is part of a long-term individual-based research project on brown bears. The study area covers approximately 13,000 km2 and is predominantly covered by intensively managed boreal forest. The altitude gradually increases from ≈150 m above sea level in the east to 850 m above sea level in the west, which is also the approximate tree line. Snow cover increases towards the north-west and with altitude and lasts approximately from late-November to April or May, mean daily temperatures are −7 °C in January and 15 °C in June [40]. During the hyperphagic period, brown bears feed on different berry species (Vaccinium myrtillus, V. vitis-idea, Empetrum hermaphoditum) [41]. The study area time zone is 1 h ahead of coordinated universal time (UTC) in winter and 2 h ahead in summer.
Subjects and devices deployed
A total of 63 brown bears (1–22 years old, 15–233 kg) were captured by darting from a helicopter, as previously described [42]. A schematic illustration of the data set is presented in Fig. 1 and a detailed overview over all individuals included in the study can be found in Additional file 1: Table S1. Captures were performed from the end of February to early July during 2010–2016. The bears were fitted with global positioning system (GPS) collars (Vectronics Aerospace GmbH, Berlin, Germany), very high frequency (VHF) abdominal implants (Telonics Inc., Mesa, AZ, USA), intraperitoneal temperature loggers (DST Centi, Star Oddi, Gardaber, Iceland), and subcutaneous HR loggers (Reveal XT, Medtronic Inc., Mounds View, Minnesota, USA). At capture, all bears were weighed with a digital spring scale and age was based on known birth year or determined from tooth sections [43]. Bears were captured and weighed in winter (late February to early March), spring (late April to May), or summer (June to early July), depending on the capture period. Bears were weighed every time they were captured and all data for HR and Tb correspond to the same year as body mass measurements. All bears, that were captured in winter were recaptured in summer, bears captured in April/May were typically only captured once that season. We associated winter data with either the body mass at winter capture or from the spring immediately after hibernation, depending on when the bear was first captured. For summer data we used the body mass measured either at summer capture or the preceding spring, depending on when the bear was last captured.
Sample sizes for each analysis. Some brown bears had only body temperature (Tb) or heart rate (HR) logging, and not both, and some were excluded from HR or Tb, due to failure of a specific logger or from one season, due to pregnancy, or in one case, killed by another bear. Some bears from summer were not available in winter, because they were killed during the autumn bear hunt
The Reveal XT preprogramed settings provided mean HR from UTC 08:00 am to UTC 08:00 pm (heart rate during daytime, HRD) and UTC 00:00 am to UTC 04:00 am (heart rate during nighttime, HRN). The heart rate means for each time period were calculated by the device from continuous ECG. These “generation 2” devices are previously described in detail [44]. Tb was recorded by the loggers at intervals ranging from 1 to 15 min for 1–3 years. We adjusted the Tb measurements to thesame time intervals as the preprogrammed HR measurements to allow for comparison between Tb and HR by averaging the Tb for night hours (TbN) and day hours (TbD). This resulted in a time series with two values per day for each physiological variable for each bear. A subset of this data, from 14 bears collected 2010–2012, was included in a study on the drivers of hibernation in brown bears [45].
We divided data on Tb and HR into a winter dataset ranging from 1 December to 23 February and a summer dataset from 31 May to 20 August. We chose these dates because, by December, the bear’s Tb had reached hibernation levels [45] and in the last week of February, several of the bears were disturbed by capture activities [46, 47]. The summer dataset started on 31 May, because bears have left the den site by that time and are active [45]. We ended the data selection on 20 August, because the legal bear hunting starts on 21 August each year in Sweden, and periods of higher human presence have been reported to affect bear physiology [48] and behavior [49]. Some bears were excluded due to sensor failures or if they were shot in autumn during the bear hunt. We also did not include data from female bears in winter if they were pregnant the same winter because brown bears have elevated Tb values during gestation [50]. For clarification and transparency reasons we included a figure to illustrate the process and sample size at initial data collection to final sample size (Fig. 1).
Accounting for activity during summer
For the active season we chose to filter the Tb data to select for inactive periods. This allowed us to compare Tb in different sized bears during inactivity, however, we could not compare the results directly to the difference in HR as the aggregation to night and day is fixed by the device. To control for locomotor activity, we merged the raw Tb dataset with the accelerometer-based activity data from the GPS collars. The dual-axis motion sensor measures acceleration across two axes with a rate of 6–8 Hz, averages values over 5-min intervals, and generates a numeric value ranging from 0 to 255. We used a previously described threshold value for Scandinavian brown bears to determine whether a bear had been active or inactive during a given 5-min interval (inactivity was when the sum of x and y values were < 50) [51]. We interpolated Tb data for each 5-min interval to match the activity data using the base function “approx” in R with the default settings. To exclude active periods from the analysis, we calculated the mean Tb for hours with < 2 “active” 5-min intervals, i.e., the bear was active a total of 0–5 min during that hour. For Tb, we had now accounted for different activity patterns and we no longer analysed day and night separately. Of the 58 complete bear-summers with Tb data, we had activity data for 53 (from 37 individual bears). In addition, six bear-summers had to be removed, because of incomplete activity data during the summer, resulting in 47 bear-summers from 35 individual bears. We did not analyse the small-scale activity patterns present during hibernation [52].
Quantification and statistical analysis
Model selection: Tb and HR
We fitted generalized additive mixed models (GAMMs) using the function “bam” and the R-packages mgcv [53] and itsadug [54]. We built different models for the following response variables: During winter: TbD and HRD (during daytime); during summer: Tb during inactivity, HRD and HRN (daytime and nighttime are defined in methods section “b”). We expected individual, as well as year-to-year, variation and used Aikake information criterion (AIC) to compare the following random structures to account for this variation: (1) random intercept for bear ID, (2) random intercept for the combination of bear and year (further referred to as YID), (3) random intercept for year, (4) random intercept for bear ID nested in year, (5) random intercept and slope for bear ID and (6) random intercept and slope for YID (Additional file 1: Tables S3–S7). We justify the combination of bear and year (YID) by the fast year-to-year growing potential in this population (i.e., even if it is the same bear ID, we consider it statistically to be a different bear). AIC was also used to do model selection on the fixed effects of the models, to compare a (1) null model with a model containing (2) a smoother for day of the year (time) and (3) a tensor product smoother for time, multiplied by the spring body mass (continuous variable), creating an interaction-like term of the two (Additional file 1: Tables S3–S7). This final model was inspected for residual autocorrelation and consequently an autoregressive model (AR1) was added. The autoregression parameter (ρ) was based on the autocorrelation factor of the standardized residuals at lag 1 and then adjusted, based on maximum likelihood values for the same model structure, by varying ρ values (± 0.3). For model interpretation, we predicted Tb and HR values for the day in winter for bears with body mass 40 kg and 120 kg. During summer, we predicted HR for day and night and Tb during inactivity, also for bears with body mass 40 kg and 120 kg.
Sex, age, and body mass are correlated in this population [27, 28]. Female bears reach asymptotic body mass at 6 years and males reach a larger mass at 12 years [27]. In our dataset, only 13 bears were older than 10 years (Fig. 2) and only six females were heavier than 100 kg. This is due to a high proportion of adult females that either gave birth or den together with offspring and thus were excluded from the data set. To avoid collinearity among the fixed terms, we did not consider age or sex. We excluded age over body mass, because we were more interested in body mass and its effects on Tb and HR. As female bears have a lower asymptotic body mass, we first included sex and body mass in an interaction-like term, but biased sample size towards younger and lighter female bears led to overfitting of the models.
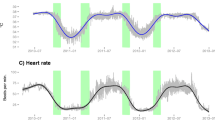
For graphical representation of the raw data sets for Tb and HR (Fig. 3), we grouped the bears by size, based on their body mass in the winter/spring, in contrast to the models, where actual body mass was used. “Small” was defined as < 60 kg, “medium” as 60–120 kg, and “large” as 120–240.
Annual variation in body temperature and heart rate (HR) across size groups; A The analysed daily mean body temperature data of brown bears in Sweden measured between 2010 and 2016 pooled in to three body mass groups: Small” (orange) was defined as < 60 kg (N = 25), “medium” (blue) as 60–120 kg (N = 11), and “large” (black) as 120–240 kg (N = 10). B Mean daytime HR of brown bears in Sweden measured between 2011 and 2016, pooled in to three body mass groups: “Small” (orange) < 60 kg (N = 32), “medium” (blue) 60–120 kg (N = 31), and “large” (black) 120–240 kg (N = 11). To illustrate the full annual cycle, the same data is partly shown twice (February to June). Sample sizes are for an individual in a given year, body mass measured in either February, May or June
Model selection: hibernation phenology
We determined the length of the hibernation period from the winter Tb dataset. For the start and end of hibernation, we used the first day that the daily mean Tb decreased below 36.4 °C for at least 7 consecutive days in autumn and the first day above 36.7 °C when remaining at this threshold for at least 7 consecutive days in spring. These temperatures have been documented to be associated with hibernation start (36.47 ± 0.14 °C) and end (36.70 ± 0.15 °C) [45]. Tb data during hibernation start and end in the following spring was available during 35 bear winters over 4 years from 25 individual bears, 12 females and 13 males, ranging in body mass from 30 to 233 kg. We also expected individual, as well as year-to-year, variation regarding the bears’ hibernation phenology and tried to fit linear mixed models with bear ID and year as random components. Aikake information criterion (AICc) differences between model fitted with and without year or bear ID was exactly 2, suggesting no change in deviance, i.e. no information gain by using a random component. Both year and bear ID showed very little variation across all years and we decided not to fit any random component. We proceeded using generalized linear models with a Poisson error distribution and a logarithmic link function for three different response variables: hibernation length in days, the start of hibernation as day of the year, and the end of the hibernation as day of the year. For each response, we selected a set of a priori formulated candidate models. Some bears were recaptured during hibernation and, as this might have led to a delayed end of hibernation [37], we included winter capture as a categorical factor to the model selection for hibernation length and hibernation end; but not for start of hibernation. Further we included a factor for the year and spring body mass. The candidate model set contained a null model and all possible variable combinations. All models were compared to each other and to a null model using AICc (Additional file 1: Table S10). We averaged all models within Δ AICc 2 using model averaging from the AICcmodavg package [55]. We performed all analyses using statistical extensions available in R 3.4.2 [56].
Results
Model performance
For all GAMM models, the highest ranked models included a random intercept and slope for YID (Additional file 1: Table S3–S7, model outputs provided in Additional file 1: Table S8). This led to varying modelled values over time, which had to be interpreted graphically. To better illustrate the predicted differences between small and large bears, only model predictions of bears of 40 and 120 kg are presented in Figs. 4, 5, 6, 7 and 8. Residual autocorrelation was high and AR 1 structures were included in all models, which did not eliminate, but considerably lowered, the autocorrelation to acceptable levels (Figure S1a–e).
Heart rate (HR) in winter: Predicted mean daytime HR for hibernating brown bears with body mass of 40 kg (orange solid line), bears with body mass of 120 kg (blue dashed line), and mean smoothed values for each bear winter (grey solid line). Shaded areas display 95% confidence intervals. HR data from 1 December to 23 February
Body temperature in winter: Predicted mean daytime body temperature (Tb) for hibernating brown bears with body mass of 40 kg (orange solid line), and bears with body mass of 120 kg (blue dashed line) and mean smoothed values for each bear winter (grey solid line). Shaded areas display 95% confidence intervals. Tb from 1 December to 23 February
Daytime summer heart rate (HR): Predicted mean HR (HR) for brown bears during summer and during day time with body mass of 40 kg (orange solid line), and bears with body mass of 120 kg (blue dashed line) and mean smoothed values for each bear summer (gray solid lines). Shaded areas display 95% confidence intervals. HR data from 1 June to 21 August measured between 10:00 and 22:00
Nighttime summer heart rate (HR): Predicted mean HR (HR) for brown bears during summer and at night with body mass of 40 kg (orange solid line), and bears with body mass of 120 kg (blue dashed line) and mean smoothed values for each bear summer (grey solid line). Shaded areas display 95% confidence intervals. HR data from 1 June to 20 August measured between 02:00 and 06:00
Passive summer body temperature: Predicted daily mean body temperature (Tb) for brown bears during inactivity in summer with body mass of 40 kg (orange solid line), and bears with body mass of 120 kg (blue dashed line) and mean smoothed values for each bear summer (grey solid line). Shaded areas display 95% confidence intervals. Tb data from 1 June to 21 August
Winter
Hibernation physiology
Body temperate and HR varied on a seasonal scale, dependent on body mass (Fig. 3, Additional file 1: Table S2, Figure S2). However, the model did not predict an effect of body mass on average daytime HR during hibernation (Fig. 4), but it did predict that larger bears had a higher overall daytime Tb with increasing differences in the start and again towards the end of the hibernation period (Fig. 5). For both, HR and Tb during hibernation, the models including the interaction of day of the year and body mass were ranked highest in the model selection process (Additional file 1: Table S3 and S4), which suggests that the inclusion of body mass improved model fit.
Of the 4,165 daytime HR values, a total of 38 values (0.9%) were above 37 bpm (mean HR at den entry [45]) and 32 of these (0.8%) were from one individual. The mean daytime HR was 16 bpm (SD 5.6 bpm), ranging from 7 to 70 bpm. The mean daytime Tb during winter was 33.6 °C, ranging from 30.2 to 37.3 °C (SD 1.0 °C).
Start of hibernation
Bears entered the den on average on the 30th of October (range: 10th October–16th November). Spring body mass did not explain the variation in the initiation of hibernation. The null model was the top model with den entry as response variable, indicating that there was no difference in the timing of den entry between different body sizes (Additional file 1: Table S9).
Hibernation duration
Larger bears displayed shorter hibernation periods (indicated by an earlier den exit). For example, for bears with a spring body mass of 120 kg, the model predicted 155 days in hibernation (95% ci 133–177 days), but for bears of 40 kg the model predicted 166 days (95% ci 152–186 days). The effect of spring body mass was negative (95% ci not overlap** with 0), indicating a decrease in hibernation period with increasing body mass. Winter capture and sex had no clear effect. Models including spring body mass alone or with winter capture in addition, were all within Δ AICc 2 (Additional file 1: Table S10), did not perform substantially differently and were therefore averaged for prediction. For predictions and interpretation, we used females that had not been captured in winter.
End of hibernation
Bears exited the den on average on the 10th of April (range: 15th March–3rd May). For the timing of the end of hibernation, the models with both body mass and winter capture as fixed factors were within AICc delta 2 i.e. no clear difference in model performance (Additional file 1: Table S11). There was a strong effect of spring body mass, but not of winter capture (see ci of average model, Table 1). Larger bears (not captured) raised their Tb earlier over the Tb level defined as hibernation (Table 1). For example, the model predicted that bears of 120 kg end hibernation around April 1 (day of the year 93; 95% ci 77–111), whereas bears of 40 kg end hibernation around April 14 (day of the year 104; 95% ci 92–115). The same model predicted a 7-day (95% ci 1–17 days) delay in the end of hibernation if a bear of 40 kg was captured in the den.
Summer
During the day in summer, HR was lower in larger bears. During night, however, HR was similar among bears. The highest ranked models on the fixed effects structures for day and nighttime HR as well as passive Tb during summer included the interaction like term for day of the year and body mass (Additional file 1: Table S5–S7) indicating that there those parameters differ between body masses (see also Additional file 1: Table S2). Visual interpretation of the prediction graph of the HR model for a 120 kg and 40 kg bear indicate decreased HR during the day for larger bears, whereas a 40 kg bears would remain at a similar HR during day and night (Figs. 6 and 7). Predicted values for daytime HR had smaller confidence intervals than nighttime values (Figs. 6 and 7) Inactive Tb in summer was lower in larger than smaller bears (Fig. 8). In the beginning of summer, Tb of small and large bears were comparable but followed different trends throughout the course of summer with smaller bears having higher Tb than larger bears.
Discussion
We evaluated the effect of body mass on Tb and HR of brown bears in Scandinavia throughout their annual cycle. The smallest individuals reached lower Tb during hibernation, hibernated longer, and ended hibernation later than large bears. In contrast to Tb, HR in winter was not associated with body mass. These relationships were consistent across a range of body masses (15–233 kg). In summer, we observed the opposite pattern, with smaller bears exhibiting higher daytime HR and trending towards higher Tb. As the smallest bears (cubs still with their mothers) move the least [57], this cannot be explained by activity. We also found that body mass played an important role in some of the phenological aspects of hibernation. Although body mass did not influence date of den entrance, the smallest bears ended hibernation latest and therefore hibernated longer in total. The finding that body mass did not have an effect on den entrance date, is consistent with our previous finding that den entrance timing is primarily dependent on environmental cues [45].
Winter
The smaller bears had higher Tb in summer, but as they entered hibernation, the relationship between Tb and body size was reversed. Interestingly, this pattern was not seen for HR, which was similar across sizes (Fig. 3, Additional file 1: Figure S2, Additional file 1: Table S2). Similar HRs in winter could indicate similar hibernating metabolic rates across sizes. These results demonstrate that the difference in Tb is not dependent on heart rate and more likely attributed to the fact that smaller animals have a higher surface-to-volume ratio, resulting in higher thermal conductance [37] and poorer heat conservation [34]. This higher thermal conductance in the smaller bears results in their ability to reduce Tb to a greater extent, but HR remains similar because the range in Tb may not be large enough to cause in a difference in HR between groups. This is consistent with interspecies comparisons, where larger hibernators have slower cooling rates [34], confirming that differences can be attributed to body size, even intra-specifically. In contrast to a previous study [37], which found that captive American black bears had a higher mass-specific minimum metabolic rate, smaller brown bears did not have higher HR, but rather similar HR to larger bears, indicating a similar metabolic rate (unfortunately that study did not look at HR, and we have not measured metabolic rate). Interestingly, the smaller bears in our study did not compensate for the increased heat loss by increasing HR and thus metabolic rate. Alternatively, there could be higher MR costs for small bears due to their high thermal conductance, raising their HR to the level of the larger bears. An interesting further question would be if the extreme sinus arrythmias are more common in smaller bears.
We noted that the smallest bears exit their dens last, with several possible explanations; 1) they have smaller fat reserves [60] and must hibernate longer to conserve energy until food availability increases. In contrast to larger bears which have larger fat reserves [60], allowing them to better withstand harsh weather and search for rare, protein-rich food, such as ungulate carrion or weakened moose (Alces alces) [39, 41, 61]., or 2) their higher critical temperature (TA) is higher than that of the larger bears, so they need a higher ambient temperature before being triggered to emerge in spring (ambient temperature is a driver of den emergence [62]).. However, a study examining the lower critical temperature across black bear body masses of 40–120 kg did not find a significant effect of body mass on lower critical temperature, possibly because of the smaller number of bears or alternatively the higher minimum metabolic rate in smaller hibernating bears might compensate for the increased conductance [37]. This contrasts to our finding that heart rate was not determined by body size during hibernation.
The difference in Tb between the larger and smaller bears also did not increase during the duration of the winter, which would be expected if the differences were purely due to increased thermal conductance and heat loss in smaller bears. HR did not differ and was stable between the two groups throughout the winter, even as Tb was drop**. This indicates that no active compensation was occurring. Most likely, the smaller bear’s later emergence is due to a combination and interaction between the factors discussed here.
Summer
In summer, body mass also influenced the Tb of inactive bears, and the effect seemed to increase towards the end of summer with smaller bears having higher Tb than larger bears. Our models predicted smaller bears to have higher HR than larger bears during the day. Smaller bears had similar predicted HRs between night and day. The larger bears increased their HR in the night, supporting similar HRs at night among bear sizes. Smaller bears have previously been shown to be more nocturnal than larger individuals [63], perhaps their persistent high HR is because of higher activity levels than the larger bears during daytime. An additive factor could be that, although they were foraging more at night, the small bears still needed to remain vigilant at all times, because of the possibility of intraspecific predation [64]. Additionally, day length changes substantially in the study area throughout the year with sunrise as early as 01:30 UTC and the rest of the night is represented by twilight. Brown bears in this study area have been shown to be active during twilight hours in summer [65, 66], which partly corresponds to our night-time HR definition (00:00–04:00 UTC). Consequently, this might have impacted our HR results when comparing day and night HR. The higher Tb in smaller bears, despite filtering for inactive hours, indicates a body mass dependent (rather than activity-dependent) difference.
The study had some limitations linked to lack of continuous measurements of body mass throughout the year. We had a rough estimate of body mass when analysing data on a yearly scale. Although sufficient to compare large differences in body mass, analyses on a fine scale of body mass had to be avoided. Thus, weight gain due to the hyperphagia phase in summer could not be accounted for in the analysis. Additionally, the HR analysis was limited by the preprogramed setting on the device, which gave only “day” and “night”, rather than continuous HR data. Settings for 2-min HR are now available [44], but were not available at the time of data collection. We cannot rule out that HR and Tb decrease with age and that body mass and age are confounding factors. Distribution of body weights in relation to age is presented in Fig. 7. Also, although these devices have been used with high success in both black bears and brown bears [44], a specific validation of the heart rate calculations would strength both our study and other studies. This is challenging with the current programming of the device to only record episodes of high and low heart rate, however newer models allow for real-time transmission of HR and ECG [44].
Although the literature is rife with interspecific patterns [3, 5], these patterns didn’t necessarily hold true when evaluated within a single species, the Scandinavian Brown bear. Smaller bears had higher Tb in summer and lower Tb in winter, in line with the principles of thermodynamics and surface-to-volume ratios. The large dataset available here allowed us to conduct comparisons across a range of sizes of free-ranging brown bears, where the relationship between environment and physiology were not altered, as is often the case for captive bears [67]. We conclude that the smallest bears hibernated more deeply and longer than large bears, likely from a combined effect of basic thermodynamics (high thermal conductance, due to a high surface-to-body-mass ratio), the higher need for energy savings, and a lower cost of warming up a smaller body. During summer, smaller bears had higher Tb and daytime HR, which is a finding not previously documented within a single mammalian species.
References
Whitfield J. In the beat of a heart. Life, Energy, and the Unity of Nature. Washington, DC: The National Academies Press;2006.
Schmidt-Nielsen K. Animal physiology. Adaptation and environment. 5th edn. Cambridge: Cambridge University Press;1997.
Brody S, Procter RC. Ashworth US: Growth and development. With Special Refeence to Domestic Animals. XXXIV. Basal Metabolism, Endogenous Nitrogen, Creatinine and Neutral Sulphur Excretions as Functions of Body Weight. University of Missouri Agricultural Experiment Station Research Bulletin 1934;220:1–40.
Clarke A, Rothery P. Scaling of body temperature in mammals and birds. Funct Ecol. 2008;22:58–67.
Morrison Peter R, Ryser Fred A. Weight and body temperature in mammals. Science. 1952;116:231–2.
Packard GC, Birchard GF. Traditional allometric analysis fails to provide a valid predictive model for mammalian metabolic rates. J Exp Biol. 2008;211:3581–7.
Refinetti R. Body size and metabolic rate in the laboratory rat. Exp Biol. 1989;48:291–4.
Glazier DS. Beyond the ‘3/4-power law’: variation in the intra-and interspecific scaling of metabolic rate in animals. Biol Rev. 2005;80:611–62.
Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol Rev. 2015;90:891–926.
Lovegrove BG. The evolution of endothermy in Cenozoic mammals: a plesiomorphic-apomorphic continuum. Biol Rev. 2012;87:128–62.
Ferasin L, Ferasin H, Little CJL. Lack of correlation between canine heart rate and body size in veterinary clinical practice. J Small Anim Pract. 2010;51:412–8.
Lamb AP, Meurs KM, Hamlin RL. Correlation of heart rate to body weight in apparently normal dogs. J Vet Cardiol. 2010;12:107–10.
Schwarzwald CC, Kedo M, Birkmann K, Hamlin RL. Relationship of heart rate and electrocardiographic time intervals to body mass in horses and ponies. J Vet Cardiol. 2012;14:343–50.
Menzies AK, Studd EK, Seguin JL, Derbyshire RE, Murray DL, Boutin S, Humphries MM. Activity, heart rate, and energy expenditure of a cold-climate mesocarnivore, the Canada lynx (Lynx canadensis). Can J Zool. 2022;100:261–72.
Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res. 1970;4:160–7.
Geiser F. Evolution of daily torpor and hibernation in birds and mammals: importance of body size. Clin Exp Pharmacol Physiol. 1998;25:736–9.
Mills SH, South FE. Central regulation of temperature in hibernation and normothermia. Cryobiology. 1972;9:393–403.
Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol. 2004;141:317–29.
Bieber C, Lebl K, Stalder G, Geiser F, Ruf T. Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct Ecol. 2014;28:167–77.
French AR. Allometries of the durations of torpid and euthermic intervals during mammalian hibernation: A test of the theory of metabolic control of the timing of changes in body temperature. J Comp Physiol - B Biochem Syst Environ Physiol. 1985;156:13–9.
Humphries MM, Thomas DW, Kramer DL. The role of energy availability in Mammalian hibernation: a cost-benefit approach. Physiol Biochem Zool. 2003;76:165–79.
Geiser F, Ruf T. Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol Zool. 1995;68:935–66.
Malan A. Is the torpor-arousal cycle of hibernation controlled by a non-temperature-compensated circadian clock? J Biol Rhythms. 2010;25:166–75.
Mahlert B, Gerritsmann H, Stalder G, Ruf T, Zahariev A, Blanc S, Giroud S. Implications of being born late in the active season for growth, fattening, torpor use, winter survival and fecundity. eLife 2018;7:e31225.
Buck CL. Life in the fast lane. Elife. 2018;7:e35029.
Cattet MRL, Caulkett NA, Obbard ME, Stenhouse GB. A body-condition index for ursids. Can J Zool. 2002;80:1156–61.
Swenson J, Adamič M, Huber D, Stokke S. Brown bear body mass and growth in northern and southern Europe. Oecologia. 2007;153:37–47.
Zedrosser A, Dahle B, Swenson JE. Population density and food conditions determine adult female body size in brown bears. J Mammal. 2006;87:510–8.
Painer J, Zedrosser A, Arnemo JM, Fahlman A, Brunberg S, Segerstrom P, Swenson JE. Effects of different doses of medetomidine and tiletamine-zolazepam on the duration of induction and immobilization in free-ranging yearling brown bears (Ursus arctos). Can J Zool. 2012;90:753–7.
Fahlman Å, Arnemo JM, Swenson JE, Pringle J, Brunberg S, Nyman G. Physiologic evaluation of capture and anesthesia with Medetomidine–Zolazepam–Tiletamine in Brown Bears (Ursus arctos). J Zoo Wildl Med. 2011;42:1–11.
Chayama Y, Ando L, Tamura Y, Miura M, Yamaguchi Y. Decreases in body temperature and body mass constitute pre-hibernation remodelling in the Syrian golden hamster, a facultative mammalian hibernator. R Soc Open Sci. 2016;3:160002.
Zervanos SM, Maher CR, Florant GL. Effect of body mass on hibernation strategies of woodchucks (Marmota monax). Integr Comp Biol. 2014;54:443–51.
Tøien Ø, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: independence of metabolic suppression from body temperature. Science. 2011;331:906–9.
Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–74.
Watts PD, Øritsland NA, Jonkel C, Ronald K. Mammalian hibernation and the oxygen consumption of a denning black bear (Ursus americanas). Comp Biochem Physiol A: Mol Integr Physiol. 1981;69:121–3.
Heldmaier G. Life on low flame in hibernation. Science. 2011;331:866–7.
Tøien Ø, Blake J, Barnes BM. Thermoregulation and energetics in hibernating black bears: metabolic rate and the mystery of multi-day body temperature cycles. J Comp Physiol B. 2015;185:447–61.
Boyles JG, Dunbar MB, Storm JJ, Brack V Jr. Energy availability influences microclimate selection of hibernating bats. J Exp Biol. 2007;210:4345–50.
Manchi S, Swenson JE. Denning behaviour of Scandinavian brown bears Ursus arctos. Wildl Biol. 2005;11:123–32.
Elfström M, Swenson JE, Ball JP. Selection of denning habitats by Scandinavian brown bears Ursus arctos. Wildl Biol. 2008;14:176–87.
Stenset NE, Lutnæs PN, Bjarnadóttir V, Dahle B, Fossum KH, Jigsved P, Johansen T, Neumann W, Opseth O, Rønning O, et al. Seasonal and annual variation in the diet of brown bears Ursus arctos in the boreal forest of southcentral Sweden. Wildl Biol. 2016;22:107–16.
Arnemo JM, Evans A. Biomedical protocols for free-ranging brown bears, wolves, wolverines and Lynx. In Wildlife Health. pp. 16. Evenstad: Inland Norway University of Applied Sciences; 2017:16.
Willey CH. Aging black bears from first premolar tooth sections. J Wildl Manag. 1974;38:97–100.
Laske TG, Evans AL, Arnemo JM, Iles TL, Ditmer MA, Fröbert O, Garshelis DL, Iaizzo PA. Development and utilization of implantable cardiac monitors in free-ranging American black and Eurasian brown bears: system evolution and lessons learned. Anim Biotelemetry. 2018;6:13.
Evans AL, Singh NJ, Friebe A, Arnemo JM, Laske TG, Fröbert O, Swenson JE, Blanc S. Drivers of hibernation in the brown bear. Front Zool. 2016;13:7.
Evans AL, Sahlén V, Støen OG, Fahlman A, Brunberg S, Madslien K, Fröbert O, Swenson JE, Arnemo JM. Capture, Anesthesia, and Disturbance of Free-Ranging Brown Bears (Ursus arctos) during Hibernation. PLoS ONE. 2012;7:e40520.
Evans AL, Singh NJ, Fuchs B, Blanc S, Friebe A, Laske TG, Frobert O, Swenson JE, Arnemo JM. Physiological reactions to capture in hibernating brown bears. Conserv Physiol. 2016;4:cow061.
Støen OG, Ordiz A, Evans AL, Laske TG, Kindberg J, Fröbert O, Swenson JE, Arnemo JM. Physiological evidence for a human-induced landscape of fear in brown bears (Ursus arctos). Physiol Behav. 2015;152:244–8.
Ordiz A, Støen O-G, Sæbø S, Kindberg J, Delibes M, Swenson JE. Do bears know they are being hunted? Biol Cons. 2012;152:21–8.
Friebe A, Evans AL, Arnemo JM, Blanc S, Brunberg S, Fleissner G, Swenson JE, Zedrosser A. Factors affecting date of implantation, parturition, and den entry estimated from activity and body temperature in free-ranging brown bears. PLoS ONE. 2014;9:e101410.
Gervasi V, Brunberg S, Swenson JE. An individual-based method to measure animal activity levels: a test on brown bears. Wildl Soc Bull. 2006;34:1314–9.
Friebe A, Zedrosser A, Swenson JE. Detection of pregnancy in a hibernator based on activity data. Eur J Wildl Res. 2013;59:731–41.
Wood S. MGCV: mixed GAM computation vehicle with GCV/AIC/REML smoothness estimation. R package version 1.8–17. In R package version, vol. 1.8–17; 2017.
van Rij J, Wieling M, Baayen RH, van Rijn H. itsadug: Interpreting time series and autocorrelated data using GAMMs. R package version 2.3 vol. 2.3; 2016.
Mazerolle MJ. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.2–1. 2019.
R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2014.
Bogdanović N, Hertel AG, Zedrosser A, Paunović M, Plećaš M, Ćirović D. Seasonal and diel movement patterns of brown bears in a population in southeastern Europe. Ecol Evol. 2021;11:15972–83.
Jessen N, Nielsen TS, Vendelbo MH, Viggers R, Støen O-G, Evans AL, Frøbert O. Pronounced expression of the lipolytic inhibitor G0/G1 Switch Gene 2 (G0S2) in adipose tissue from brown bears (Ursus arctos) prior to hibernation. Physiol Rep. 2016;4:e12781.
Nowack J, Giroud S, Arnold W, Ruf T. Muscle non-shivering thermogenesis and its role in the evolution of endothermy. Front Physiol. 2017;8:889.
Morrison P. Some interrelations between weight and hibernation function. Bull Museum Comp Zool. 1960;124:75–91.
Dahle B, Swenson JE. Seasonal range size in relation to reproductive strategies in brown bears Ursus arctos. J Anim Ecol. 2003;72:660–7.
Evans AL, Singh NJ, Friebe A, Arnemo JM, Laske TG, Fröbert O, Swenson JE, Blanc S. Drivers of hibernation in the brown bear. Front Zool. 2016;13:1–14.
Hertel AG, Swenson JE, Bischof R. A case for considering individual variation in diel activity patterns. Behav Ecol. 2017;28:1524–31.
Swenson JE, Dahle B, Sandegren F. Intraspecific predation in Scandinavian brown bears older than cubs-of-the-year. Ursus. 2001;12:81–91.
Ordiz A, Kindberg J, Sæbø S, Swenson JE, Støen O-G. Brown bear circadian behavior reveals human environmental encroachment. Biol Cons. 2014;173:1–9.
Moe TF, Kindberg J, Jansson I, Swenson JE. Importance of diel behaviour when studying habitat selection: examples from female Scandinavian brown bears (Ursus arctos). Can J Zool. 2007;85:518–25.
Hissa R, Siekkinen J, Hohtola E, Saarela S, Hakala A, Pudas J. Seasonal patterns in the physiology of the European brown bear (Ursus arctos) in Finland. Comp Biochem Physiol A Mol Integr Physiol. 1994;109:781–91.
Acknowledgements
The authors want to thank Ms. Renate Hengsberger for her support with formatting and editing of the manuscript, as well as for literature search.
Funding
The Scandinavian Brown Bear Project is supported by the Norwegian Environment Agency, the Swedish Environmental Protection Agency, the Austrian Science Fund, the Swedish Association for Hunting and Wildlife Management, and the Research Council of Norway. The Norwegian Environment Agency, Norwegian-American Fulbright Commission, the American Scandinavian Foundation, and the Morris Animal Foundation provided salary and stipend support for Dr. Evans during the study and writing periods. NJS was funded by the Beyond Moose Programme of the Swedish Environmental Protection Agency. This study was also supported by the Lundbeck Foundation and the Augustinus Foundation. The temperature loggers were provided by the Hubert Curien Institute, Strasbourg, France. The HR loggers were provided by Medtronic Inc. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, with the exception of Tim Laske, who is an employee of Medtronic Inc. SG was financially supported by the Austrian Science Fund (FWF, Grant No. P31577-B25) and the University of Veterinary medicine Vienna. This is scientific paper no. XXX from the Scandinavian Brown Bear Research Project.
Author information
Authors and Affiliations
Contributions
ALE, SB, OF, JMA, JES initiated the study and designed the experiments. ALE, AF, SB, OF, JMA, TGL, BF contributed during fieldwork and data collection. Equipment was provided by SB, TGL, JMA. Data management was done by BF and AF. Data analysis and preparation of figures was done initially by BF, with contributions and revisions from AT, NJS and ALE. Physiological interpretation of results was done by ALE, SG, BF, AT and SB. ALE and BF drafted the manuscript, JMA and SG critically revised the manuscript, and all authors participated in revisions, approved the final manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical statement
All captures were approved by the Ethical Committee on Animal Experiments, Uppsala, Sweden (Application Numbers C47/9, C7/12, C18/15, C212/9, and C268/12) and the Swedish Environmental Protection Agency. All methods were carried out in accordance with relevant guidelines and regulations including the biomedical protocols for capture of brown bears [42].
Data statement
Data requests should be addressed to Alina L Evans. HR and Tb data from brown bears and other species are stored at the Umeå Center for Wireless Remote Animal Monitoring (http://www.slu.se/wram).
Competing interests
Timothy Laske is an employee of Medtronic Inc. Remaining Authors have no Competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. This supplemental material included the referenced material including autocorrelation plots of the models, an overview plot of body temperature and heart rate in relation to body size, overview table of each individual used in the analysis, overview table of monthly average body temperature and heart rate, AIC model selection tables and summary statistics for the highest ranked models.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Evans, A.L., Fuchs, B., Singh, N.J. et al. Body mass is associated with hibernation length, body temperature, and heart rate in free-ranging brown bears. Front Zool 20, 27 (2023). https://doi.org/10.1186/s12983-023-00501-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12983-023-00501-3