Abstract
Background
Postoperative cognitive dysfunction (POCD) is a common neurological complication of anesthesia and surgery in aging individuals. Neuroinflammation has been identified as a hallmark of POCD. However, safe and effective treatments of POCD are still lacking. Itaconate is an immunoregulatory metabolite derived from the tricarboxylic acid cycle that exerts anti-inflammatory effects by activating the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. In this study, we investigated the effects and underlying mechanism of 4-octyl itaconate (OI), a cell-permeable itaconate derivative, on POCD in aged mice.
Methods
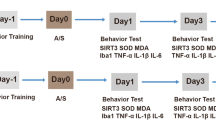
A POCD animal model was established by performing aseptic laparotomy in 18-month-old male C57BL/6 mice under isoflurane anesthesia while maintaining spontaneous ventilation. OI was intraperitoneally injected into the mice after surgery. Primary microglia and neurons were isolated and treated to lipopolysaccharide (LPS), isoflurane, and OI. Cognitive function, neuroinflammatory responses, as well as levels of gut microbiota and their metabolites were evaluated. To determine the mechanisms underlying the therapeutic effects of OI in POCD, ML385, an antagonist of Nrf2, was administered intraperitoneally. Cognitive function, neuroinflammatory responses, endogenous neurogenesis, neuronal apoptosis, and Nrf2/extracellular signal-related kinases (ERK) signaling pathway were evaluated.
Results
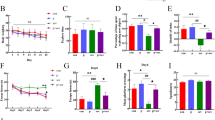
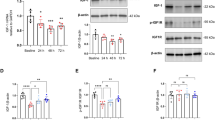
Our findings revealed that OI treatment significantly alleviated anesthesia/surgery-induced cognitive impairment, concomitant with reduced levels of the neuroinflammatory cytokines IL-1β and IL-6, as well as suppressed activation of microglia and astrocytes in the hippocampus. Similarly, OI treatment inhibited the expression of IL-1β and IL-6 in LPS and isoflurane-induced primary microglia in vitro. Intraperitoneal administration of OI led to alterations in the gut microbiota and promoted the production of microbiota-derived metabolites associated with neurogenesis. We further confirmed that OI promoted endogenous neurogenesis and inhibited neuronal apoptosis in the hippocampal dentate gyrus of aged mice. Mechanistically, we observed a decrease in Nrf2 expression in hippocampal neurons both in vitro and in vivo, which was reversed by OI treatment. We found that Nrf2 was required for OI treatment to inhibit neuroinflammation in POCD. The enhanced POCD recovery and promotion of neurogenesis triggered by OI exposure were, at least partially, mediated by the activation of the Nrf2/ERK signaling pathway.
Conclusions
Our findings demonstrate that OI can attenuate anesthesia/surgery-induced cognitive impairment by stabilizing the gut microbiota and activating Nrf2 signaling to restrict neuroinflammation and promote neurogenesis. Boosting endogenous itaconate or supplementation with exogenous itaconate derivatives may represent novel strategies for the treatment of POCD.
Similar content being viewed by others
Introduction
Postoperative cognitive dysfunction (POCD) is defined as long-term cognitive impairment that occurs weeks to months after surgery. The incidence of POCD in major non-cardiac surgery patients aged > 65 years has been reported to be 25.8% at one week and 9.9% at three months postoperatively [1,2,3,4]. Although the exact pathophysiology behind the development of POCD is not fully understood, it is believed to be caused by the following mechanisms: blood–brain barrier damage, neuroinflammatory responses, abnormal synaptic transmission, neuronal apoptosis, oxidative stress, abnormal amyloid-beta deposition and Tau protein phosphorylation, polymorphisms of the apolipoprotein E gene, and abnormal energy metabolism [5,6,7]. In addition, long-standing geriatric POCD can be a consequence of Alzheimer’s disease, decreasing quality of life and imposing a significant economic burden on families and society [8]. With the growing elderly population worldwide, the need for surgical procedures is rapidly increasing, leading to a high prevalence of POCD. However, our understanding and diagnosis of POCD still encounter numerous challenges. Therefore, there is an urgent need to explore the pathogenesis of POCD from a new perspective and identify new targets for drug therapy.
Itaconate is an endogenous metabolite derived from the tricarboxylic acid cycle and is produced by cis-aconitic acid decarboxylation catalyzed by immune response gene 1 (IRG1) in the mitochondrial matrix [9]. Itaconate is an immunoregulatory metabolite that activates the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. In recent years, it has been shown that itaconate can attenuate neuroinflammation and exert dopamine neuroprotection in Parkinson’s disease through inhibition of NLR-family pyrin domain-containing protein 3 (NLRP3) inflammasome. The mechanism of this neuroprotective effect of itaconate involves the scaffold protein p62/Nrf2/Heme oxygenase 1 (HO-1)/Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) axis pathway in microglia [ The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number (s) can be found below: NCBI BioProject (https://www.ncbi.nlm.nih.gov/sra/PRJNA1078590) and MetaboLights repository (https://www.ebi.ac.uk/metabolights/editor/study/MTBLS9593). Other datasets used and analyzed during this study can be obtained from the corresponding author upon reasonable request. Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-20181. J Alzheimers Dis. 2018;66(1):1–10. Leslie M. The post-op brain. Science. 2017;356(6341):898–900. Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351(9106):857–61. Wei P, Zheng Q, Liu H, et al. Nicotine-induced neuroprotection against cognitive dysfunction after partial hepatectomy involves activation of BDNF/TrkB signaling pathway and inhibition of NF-kappaB signaling pathway in aged rats. Nicotine Tob Res. 2018;20(4):515–22. Cao L, Wang K, Gu T, et al. Association between APOE epsilon 4 allele and postoperative cognitive dysfunction: a meta-analysis. Int J Neurosci. 2014;124(7):478–85. Cascella M, Bimonte S. The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction. Neural Regen Res. 2017;12(11):1780–5. Feinkohl I, Winterer G, Pischon T. Diabetes is associated with risk of postoperative cognitive dysfunction: A meta-analysis. Diabetes Metab Res Rev. 2017;33(5): e2884. Wu Z, Zhang M, Zhang Z, et al. Ratio of beta-amyloid protein (Abeta) and Tau predicts the postoperative cognitive dysfunction on patients undergoing total hip/knee replacement surgery. Exp Ther Med. 2018;15(1):878–84. Mills EL, Ryan DG, Prag HA, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113–7. **a N, Madore V, Albalakhi A, et al. Microglia-dependent neuroprotective effects of 4-octyl itaconate against rotenone-and MPP+-induced neurotoxicity in Parkinson’s disease. Sci Rep. 2023;13(1):15539. Sun G, Zhang R, Liu C, et al. Itaconate attenuates neuroinflammation and exerts dopamine neuroprotection in Parkinson’s disease through inhibiting NLRP3 inflammasome. Brain Sci. 2022;12(9):1255. Lampropoulou V, Sergushichev A, Bambouskova M, et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24(1):158–66. Swain A, Bambouskova M, Kim H, et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nat Metab. 2020;2(7):594–602. Meiser J, Kraemer L, Jaeger C, et al. Itaconic acid indicates cellular but not systemic immune system activation. Oncotarget. 2018;9(63):32098–107. Cordes T, Lucas A, Divakaruni AS, et al. Itaconate modulates tricarboxylic acid and redox metabolism to mitigate reperfusion injury. Mol Metab. 2020;32:122–35. Liu R, Gong Y, **a C, et al. Itaconate: A promising precursor for treatment of neuroinflammation associated depression. Biomed Pharmacother. 2023;167: 115521. Zhang P, Wang Y, Yang W, et al. 4-Octyl itaconate regulates immune balance by activating Nrf2 and negatively regulating PD-L1 in a mouse model of sepsis. Int J Biol Sci. 2022;18(16):6189–209. Huang B, Wu H, Zheng L, et al. Activation of Nrf2 signaling by 4-octyl itaconate attenuates the cartilaginous endplate degeneration by inhibiting E3 ubiquitin ligase ZNF598. Osteoarthritis Cartilage. 2023;31(2):213–27. Yang W, Wang Y, Huang Y, et al. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to promote cuproptosis in colorectal cancer. Biomed Pharmacother. 2023;159: 114301. Singh A, Venkannagari S, Oh KH, et al. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem Biol. 2016;11(11):3214–25. Hu Z, Xu D, Meng H, et al. 4-octyl itaconate protects against oxidative stress-induced liver injury by activating the Nrf2/Sirt3 pathway through AKT and ERK1/2 phosphorylation. Biochem Pharmacol. 2024;220: 115992. Cui Y, Zhang Z, Lv M, et al. Chromatin target of protein arginine methyltransferases alleviates cerebral ischemia/reperfusion-induced injury by regulating RNA alternative splicing. iScience. 2024;27(1):108688. Zhao R, Zhou X, Zhao Z, et al. Farrerol alleviates cerebral ischemia-reperfusion injury by promoting neuronal survival and reducing neuroinflammation. Mol Neurobiol. 2024. https://doi.org/10.1007/s12035-024-04031-9. Kong X, Yao X, Ren J, et al. tDCS regulates ASBT-3-OxoLCA-PLOD2-PTEN signaling pathway to confer neuroprotection following rat cerebral ischemia-reperfusion injury. Mol Neurobiol. 2023;60(11):6715–30. Lai Z, Shan W, Li J, et al. Appropriate exercise level attenuates gut dysbiosis and valeric acid increase to improve neuroplasticity and cognitive function after surgery in mice. Mol Psychiatry. 2021;26(12):7167–87. Qiu G, Wang P, Rao J, et al. Dexmedetomidine inhibits paraventricular corticotropin-releasing hormone neurons that attenuate acute stress-induced anxiety-like behavior in mice. Anesthesiology. 2024. https://doi.org/10.1097/ALN.0000000000004982. Kong X, Hu W, Cui Y, et al. Transcranial direct-current stimulation regulates MCT1-PPA-PTEN-LONP1 signaling to confer neuroprotection after rat cerebral ischemia-reperfusion injury. Mol Neurobiol. 2022;59(12):7423–38. Mellios N, Feldman DA, Sheridan SD, et al. MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol Psychiatry. 2018;23(4):1051–65. Nguyen BT, Shin EJ, Jeong JH, et al. Mountain-cultivated ginseng protects against cognitive impairments in aged GPx-1 knockout mice via activation of Nrf2/ChAT/ERK signaling pathway. J Ginseng Res. 2023;47(4):561–71. Dilmen OK, Meco BC, Evered LA, et al. Postoperative neurocognitive disorders: a clinical guide. J Clin Anesth. 2024;92: 111320. Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, et al. State of the clinical science of perioperative brain health: report from the American society of anesthesiologists brain health initiative summit 2018. Br J Anaesth. 2019;123(4):464–78. Vacas S, Cole DJ, Cannesson M. Cognitive decline associated with anesthesia and surgery in older patients. JAMA. 2021. https://doi.org/10.1001/jama.2021.4773. Skvarc DR, Berk M, Byrne LK, et al. Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev. 2018;84:116–33. Evered L, Atkins K, Silbert B, et al. Acute peri-operative neurocognitive disorders: a narrative review. Anaesthesia. 2022;77(Suppl 1):34–42. Yang T, Velagapudi R, Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. 2020;21(11):1319–26. Wei P, Yang F, Zheng Q, et al. The potential role of the NLRP3 inflammasome activation as a link between mitochondria ROS generation and neuroinflammation in postoperative cognitive dysfunction. Front Cell Neurosci. 2019;13:73. Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7. Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68(3):360–8. Yang T, Xu G, Newton PT, et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br J Anaesth. 2019;122(3):350–60. Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986–95. Takazawa T, Horiuchi T, Orihara M, et al. Prevention of postoperative cognitive dysfunction by minocycline in elderly patients after total knee arthroplasty: a randomized, double-blind, placebo-controlled clinical trial. Anesthesiology. 2023;138(2):172–83. McFadden BA, Purohit S. Itaconate, an isocitrate lyase-directed inhibitor in Pseudomonas indigofera. J Bacteriol. 1977;131(1):136–44. Ruetz M, Campanello GC, Purchal M, et al. Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science. 2019;366(6465):589–93. Li Y, Xu Y, Li W, et al. Itaconate inhibits SYK through alkylation and suppresses inflammation against hvKP induced intestinal dysbiosis. Cell Mol Life Sci. 2023;80(11):337. Pan W, Zhao J, Wu J, et al. Dimethyl itaconate ameliorates cognitive impairment induced by a high-fat diet via the gut-brain axis in mice. Microbiome. 2023;11(1):30. Luo Z, Sheng Z, Hu L, et al. Targeted macrophage phagocytosis by Irg1/itaconate axis improves the prognosis of intracerebral hemorrhagic stroke and peritonitis. EBioMedicine. 2024;101: 104993. Li W, Li Y, Kang J, et al. 4-octyl itaconate as a metabolite derivative inhibits inflammation via alkylation of STING. Cell Rep. 2023;42(3): 112145. **ong J, Lu DL, Chen BQ, et al. Dimethyl itaconate reduces cognitive impairment and neuroinflammation in APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer’s disease. Neuromol Med. 2023;25(2):179–92. Liu Y, **a P, Zong S, et al. Inhibition of Alzheimer’s disease by 4-octyl itaconate revealed by RNA-seq transcriptome analysis. Eur J Pharmacol. 2024;968: 176432. Zhang Z, Li J, Wang B, et al. Ubiquitin-specific protease 22 promotes neural stem cells stemness maintenance and adult hippocampal neurogenesis, contributing to cognitive recovery following traumatic brain injury. Neuroscience. 2022;496:219–29. Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17(4):385–95. Huang J, Kin Pong U, Yang F, et al. Human pluripotent stem cell-derived ectomesenchymal stromal cells promote more robust functional recovery than umbilical cord-derived mesenchymal stromal cells after hypoxic-ischaemic brain damage. Theranostics. 2022;12(1):143–66. Sun J, Zhou X, Wu J, et al. Ligustilide enhances hippocampal neural stem cells activation to restore cognitive function in the context of postoperative cognitive dysfunction. Eur J Neurosci. 2021;54(3):5000–15. Lang HL, Zhao YZ, **ao RJ, et al. Small extracellular vesicles secreted by induced pluripotent stem cell-derived mesenchymal stem cells improve postoperative cognitive dysfunction in mice with diabetes. Neural Regen Res. 2023;18(3):609–17. Boorman E, Killick R, Aarsland D, et al. NRF2: an emerging role in neural stem cell regulation and neurogenesis. Free Radic Biol Med. 2022;193(Pt 1):437–46. Chen Y, Dong J, Gong L, et al. Fucoxanthin, a marine derived carotenoid, attenuates surgery-induced cognitive impairments via activating Akt and ERK pathways in aged mice. Phytomedicine. 2023;120: 155043. Song Z, Zhang Y, Zhang H, et al. Isoliquiritigenin triggers developmental toxicity and oxidative stress-mediated apoptosis in zebrafish embryos/larvae via Nrf2-HO1/JNK-ERK/mitochondrion pathway. Chemosphere. 2020;246: 125727. He F, Ye B, Wu X, et al. CHFR promotes metastasis of human gastric carcinoma by activating AKT and ERK via NRF2- ROS axis. BMC Gastroenterol. 2023;23(1):114. Liu N, Liang Y, Wei T, et al. The role of ferroptosis mediated by NRF2/ERK-regulated ferritinophagy in CdTe QDs-induced inflammation in macrophage. J Hazard Mater. 2022;436: 129043. Not applicable Support for this study was provided from the National Natural Science Foundation of China (Nos.82101255), the Natural Science Foundation of Shandong Province (ZR2020QH291 and ZR2020MH126), the Scientific Research Foundation of Qilu Hospital of Shandong University (QDKY2023ZD02), the Qingdao Key Health Discipline Development Fund (QDZDZK2022094) and the Qingdao Outstanding Health Professional Development Fund (2023). XK and PW performed the experiment and wrote the manuscript. WL, CL, XL, performed the in vivo experiments. LX, HF, KS performed analyzed the data. PW and XK reviewed the statistical analysis and updated the figures in the revised manuscript. JL and PW conceived the project and revised the manuscript. All animal experiments were conducted in compliance with National Institutes of Health Guidelines and were approved by the institutional animal care and use committee of the Qilu hospital (Qingdao) (KYDWLL-202107). All authors concur with the submission. The authors declare that they have no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data. Kong, X., Lyu, W., Lin, X. et al. Itaconate alleviates anesthesia/surgery-induced cognitive impairment by activating a Nrf2-dependent anti-neuroinflammation and neurogenesis via gut-brain axis.
J Neuroinflammation 21, 104 (2024). https://doi.org/10.1186/s12974-024-03103-w Received: Accepted: Published: DOI: https://doi.org/10.1186/s12974-024-03103-wAvailability of data and materials
References
Acknowledgements
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Additional information
Publisher's Note
Rights and permissions
About this article
Cite this article
Keywords