Abstract
In this study, we investigated CD70 as a promising target for renal cell carcinoma (RCC) therapy and developed a potent chimeric antigen receptor T (CAR-T) cells for potential clinical testing. CD70, found to be highly expressed in RCC tumors, was associated with decreased survival. We generated CAR-T cells expressing VHH sequence of various novel nanobodies from immunized alpaca and a single-chain variable fragment (scFv) derived from human antibody (41D12). In our in vitro experiments, anti-CD70 CAR-T cells effectively eliminated CD70-positive tumor cells while sparing CD70-negative cells. The nanobody-based CAR-T cells demonstrated significantly higher production of cytokines such as IL-2, IFN-γ and TNF-ɑ during co-culture, indicating their potential for enhanced functionality. In xenograft mouse model, these CAR-T cells exhibited remarkable anti-tumor activity, leading to the eradication of RCC tumor cells. Importantly, human T cell expansion after infusion was significantly higher in the VHH groups compared to the scFv CAR-T group. Upon re-challenging mice with RCC tumor cells, the VHH CAR-T treated group remained tumor-free, suggesting a robust and long-lasting anti-tumor response. These findings provide strong support for the potential of nanobody-based CD70 CAR-T cells as a promising therapeutic option for RCC. This warrants further development and consideration for future clinical trials and applications.
Similar content being viewed by others
Renal cell carcinoma (RCC) is the most common form of kidney cancer and ranks as the fourteenth most prevalent cancer worldwide. In 2020, more than 400,000 new RCC cases were diagnosed, resulting in over 179,000 deaths [1]. Advanced RCC often shows resistance to chemotherapy, radiotherapy, and immunotherapy. Currently, the FDA has approved various treatments, including receptor tyrosine kinase inhibitors (RTKis) like VEGF/VEGFR inhibitors, mTOR inhibitors, immune checkpoint inhibitors (ICIs), and cytokines such as interferon-alpha (IFN-α) and interleukin-2 (IL-2). In clinical practice, first-line treatments for RCC often involve combining ICIs with RTKis or using a combination of two ICIs. However, for patients who do not respond to or relapse after these therapies, the limited treatment options highlight the need for innovative and mechanistically distinct treatments.
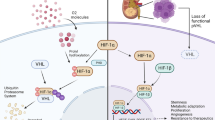
CD70, as a ligand of CD27, is a surface-expressed member of the tumor necrosis factor receptor superfamily [2]. CD70 expression is primarily found in a small subset of lymphoid lineage cells, including highly activated B and T cells, mature dendritic cells, and natural killer cells [3]. It is rarely expressed in normal non-hematopoietic tissues, and even in lymphoid tissues like the thymus, spleen, and lymph nodes, CD70 + cells are infrequent. Interestingly, CD70 is frequently expressed on T- and B-cell lymphomas and a significant fraction of solid tumors, such as thymic carcinoma, glioblastoma, renal cell carcinoma, osteosarcoma, and nasopharyngeal carcinoma [4,5,6,7,8]. Growing evidence suggests that tumor-associated CD70 expression may have immune suppressive properties [9, 10]. Therefore, there are ongoing efforts to target CD70 for therapeutic purposes, utilizing approaches like antibodies and cytotoxic antibody conjugates. Some of these candidates have entered clinical trials, such as cusatuzumab [11,12,13,14].
Chimeric antigen receptor T (CAR-T) cell immunotherapy has revolutionized cancer treatment by reprogramming a patient’s T cells to target and eliminate cancer cells. While CAR-T cell therapy has been highly effective against hematological malignancies [22]. In addition, CRISPR Therapeutics has developed another allogeneic CD70 CAR T cells (CTX-130), which has shown early signs of clinical activity in advanced RCC patients, according to findings from the phase 1 COBALT-RCC trial (NCT04438083) [36]. Currently, both ALLO-316 and CTX-130 represent the rapidly advancing CD70 CAR-T candidates for RCC treatment in clinical development. However, it is essential to note that despite significant advancement in allogeneic CAR-T therapies, they still exhibit lower efficacy and raise more safety concern than the autologous counterparts.
Autologous CD19 CAR-T cell products have been the most successful CAR-T cell therapy and have led the FDA approvals of three such products: tisagenlecleucel (Kymriah, Novartis), axicabtagene ciloleucel (Yescarta, Kite Pharmaceuticals), and brexucabtagene autoleucel (Tecartus, Kite Pharmaceuticals) [37,38,39,40]. Autologous CAR T cells have shown robust anti-tumor activity against hematological malignancies targeting BCMA, CD20, CD22, and CD30 [41,42,43,44]. While autologous CD19-CAR T cells have clinical and economical limitations, including challenges associated with leukapheresis, manufacturing and efficacy in heavily pre-treated patient population, they have shown a consistent safety profile and durable responses [45]. On the other hand, allogeneic CAR-T cells pose their own set of challenges, such as the potential to induce graft-versus-host-disease and the risk of immune-mediated rejection by the host [46]. Importantly, the regulatory path and the Chemistry, Manufacturing, and Controls (CMC) requirements for autologous CAR-Ts are well established. Therefore, autologous CAR-T therapies have been shown to be a more mature and safer product. We firmly believe that autologous CD70 CAR-T cell therapies will address the unmet needs of RCC patients.
In conclusion, we have successfully developed a nanobody-derived CD70-specific autologous CAR-T cell therapy for RCC treatment. Based on this research, intensive preclinical studies and CMC research led to the approval from the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) this year. Clinical trial of autologous CD70 CAR-T will conduct to address the more critical questions surrounding this novel RCC therapy.