Abstract
Background
Gene expression signatures can be used as prognostic biomarkers in various types of cancers. We aim to develop a gene signature for predicting the response to radiotherapy in glioma patients.
Methods
Radio-sensitive and radio-resistant glioma cell lines (M059J and M059K) were subjected to microarray analysis to screen for differentially expressed mRNAs. Additionally, we obtained 169 glioblastomas (GBM) samples and 5 normal samples from The Cancer Genome Atlas (TCGA) database, as well as 80 GBM samples and 4 normal samples from the GSE7696 set. The “DESeq2” R package was employed to identify differentially expressed genes (DEGs) between the normal brain samples and GBM samples. Combining the prognostic-related molecules identified from the TCGA, we developed a radiosensitivity-related prognostic risk signature (RRPRS) in the training set, which includes 152 patients with glioblastoma. Subsequently, we validated the reliability of the RRPRS in a validation set containing 616 patients with glioma from the TCGA database, as well as an internal validation set consisting of 31 glioblastoma patients from the Nanfang Hospital, Southern Medical University.
Results
Based on the microarray and LASSO COX regression analysis, we developed a nine-gene radiosensitivity-related prognostic risk signature. Patients with glioma were divided into high- or low-risk groups based on the median risk score. The Kaplan–Meier survival analysis showed that the progression-free survival (PFS) of the high-risk group was significantly shorter. The signature accurately predicted PFS as assessed by time-dependent receiver operating characteristic curve (ROC) analyses. Stratified analysis demonstrated that the signature is specific to predict the outcome of patients who were treated using radiotherapy. Univariate and multivariate Cox regression analysis revealed that the predictor was an independent predictor for the prognosis of patients with glioma. The prognostic nomograms accompanied by calibration curves displayed the 1-, 2-, and 3-year PFS and OS in patients with glioma.
Conclusion
Our study established a new nine-gene radiosensitivity-related prognostic risk signature that can predict the prognosis of patients with glioma who received radiotherapy. The nomogram showed great potential to predict the prognosis of patients with glioma treated using radiotherapy.
Similar content being viewed by others
Introduction
Glioma is the most common primary central nervous system (CNS) cancer, accounting for 78.3% of all malignant brain tumors [1,2,3]. Highly malignant gliomas have a poor prognosis, with a median PFS of less than 7 months [4, 5]. At present, the comprehensive glioma treatment strategy is based on maximal surgical resection, chemotherapy, and radiotherapy. Among them, radiotherapy has significant clinical benefits in improving outcomes for patients with glioma [6, 7]. However, despite its efficacy, 95% of patients with glioblastoma relapse after radiotherapy, with more than 80% of the relapse pattern occurring within the irradiation field [8, 9]. This suggests that radiation resistance of glioma cells may be a significant factor. Several studies have identified potential mechanisms of radio-resistance in gliomas, including glioma cell stemness, DNA damage repair, cell cycle blockade, changes to the tumor microenvironment, and regulation of autophagy [10, 11]. However, the underlying molecular mechanisms of radio-resistance remain unclear. Molecular markers, including 1p/19q co-deletion and mutations in TERT (encoding telomerase reverse transcriptase), P53, and ATRX (encoding alpha thalassemia/mental retardation syndrome X-linked chromatin remodeler), have been identified as crucial predictors of patient prognosis and treatment sensitivity [12,13,14]. Identifying biomarkers associated with radiotherapy response is essential to understanding the mechanism of glioma radio-resistance.
Some studies [15,16,17] have demonstrated the prognostic ability of gene signatures for disease prognosis, and the prediction of metastasis and recurrence in patients. And, there are also several studies concentrating on the sensitivity of radiotherapy in patients with gliomas. Lin et al. [18] developed a three-lncRNA signature to predict clinical outcomes in low-grade glioma patients after radiotherapy, which was established according to radiotherapeutic response (complete or partial responses) and divided patients into the radiotherapy-resistant group and radiotherapy-sensitive group and analyzed the differentially expressed lncRNAs. Zhang et al. [19] established a five-microRNA signature according to the association of the LGG (Low Grade Glioma) patients’ overall survival (OS) with microRNA expression. Yan et al. [20] analyzed those genes significantly associated with OS in radiotherapy patients and intersected immune-related genes to construct a 21-gene signature to identify patients with LGGs who would benefit from radiotherapy. However, there are several limitations to those studies. Firstly, most patients underwent surgical resection, and there were no observable lesions to evaluate the radiotherapy response. Second, there are many confounding factors affecting the clinical survival data, including the extent of surgical resection and chemotherapy response. It might lead to clinical response or survival data that cannot fully reflect radio-sensitivity. To address these limitations, we constructed a pair of radio-resistant and radio-sensitive cell lines in vitro, generated a microarray to obtain differentially expressed genes (DEGs) related to radiosensitivity, and combined these DEGs with PFS-related genes obtained from the TCGA database to identify radiosensitivity-related genes. In this study, we sought to identify biomarkers of radiosensitivity in patients with glioma to predict their response to radiotherapy and their PFS. We derived the M059J and M059K cell lines from two parts of the same tumor, with the former being radio-sensitive and the latter being relatively resistant, demonstrating a sensitivity difference of approximately 30-fold. Using microarray analysis, we compared the mRNA expression profiles of the two cell lines and identified several hub genes related to radiosensitivity, constructing a radiosensitivity-related prognostic risk signature (RRPRS). We evaluated the performance of the RRPRS in different cohorts, examined its predictive power in patients with glioma, and developed nomograms to guide clinical practice and predict PFS and OS in glioma patients.
Methods
Cell lines and culture
The human glioblastoma cell line M059K (CRL-2365) and M059J (CRL-2366) cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). M059K cells express normal levels of DNA-dependent protein kinase while M059J cells lack DNA-dependent protein kinase activity. M059K was radio-resistant and M059J was relatively radio-sensitive (the sensitivity of radiotherapy differed by a factor of approximately 30)[21]. M059K and M059J cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F12 medium (1:1 mixture) (GIBCO, Australia) supplemented with 10% fetal calf serum (GIBCO), 1 mM non-essential amino acid (Sigma) [Full size image
The predictive value of the RRPRS for the PFS of patients with glioma
After screening those patients who lacked radiation therapy information, 152 patients with glioblastoma and 616 patients with glioma in the TCGA database were included in training cohorts and validation cohorts, respectively, while the test cohort consisted of 31 glioblastoma patients from Nanfang Hospital, Southern Medical University (SMU-NFH). The detailed clinicopathological characteristics of the patients are shown in Table 1. Univariate Cox regression analysis showed that age, IDH (encoding isocitrate dehydrogenase) mutation status, 1p19q codeletion status, O6-methylguanine (O6-MeG)-DNA methyltransferase (MGMT) status, and RRPRS risk scores correlated significantly with the PFS of patients with glioma (Table 2). Multivariate Cox regression analysis confirmed that RRPRS remained an independent prognostic factor after adjusting for other clinicopathological factors. The same results were also recapitulated in the CGGA database (Additional file 1: Table S1).
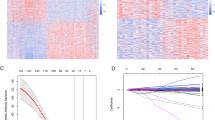
Using the median risk score as the cutoff value, the training set patients were divided into RRPRS-high-risk (n = 76) and RRPRS-low-risk groups (n = 76). The same approach was used for the validation set and test cohort. In the training set, the Kaplan–Meier survival curve showed that the PFS of the RRPRS-high-risk group was significantly shorter than that of the low-risk group (P < 0.001) (Fig. 3a). Furthermore, the model built using the RRPRS showed good performance on the ROC analysis for PFS, with AUC values of 0.747, 0.867, and 0.879 for 1-year, 2-year, and 3-year PFS, respectively (Fig. 3b). Figure 3c illustrates the risk score distribution, survival status distribution, and gene expression of each patient in the training set. The heat map showed that the high-risk group had higher expression levels of OSMR and PLK2, while the low-risk group had lower expression levels of ITPKA and L1CAM. In the validation set and SMU-NFH set, the PFS for the RRPRS-high-risk group was also shorter (P < 0.001 and P = 0.034, log-rank test) (Fig. 3d, g). The AUCs for 1-year, 2-year, and 3-year PFS were 0.778, 0.689, and 0.673 in the validation set, respectively, and 0.609 and 0.847 in the data from our center (Fig. 3e, h). The risk score, survival information, and the expression distributions of the nine genes in the patients are shown in Fig. 3f and i.
The predictive value of the RRPRS for the PFS of patients with glioma. Kaplan–Meier plots, time‐dependent ROC curves, the distributions of risk scores, survival status, and expression of radiosensitivity-related genes in the training set (a–c), validation set (d–f), and the SMU-NFH cohort (g–i). Significance for PFS analysis was calculated using a log-rank test, with the red line representing the RRPRS-high-risk group and the blue line representing the RRPRS-low-risk group. Red in the heatmap represents a high expression level and blue represents a low expression level
Radiotherapy stratification analysis
To evaluate whether the RRPRS can predict radiation therapy outcomes in patients with glioma, we performed stratified analyses in the validation set and SMU-NFH set since most patients from the TCGA datasets had received radiotherapy according to the clinical information shown in Table 1. The PFS in the different risk groups of patients with glioma who received radiotherapy was statistically significant (Fig. 4a–c), in contrast to the non-radiotherapy group (Fig. 4d–f). To further verify the effectiveness of the predictor, the CGGA database was used to validate the prediction efficacy of RRPRS. Since the data on PFS of patients were lacking in the CGGA database, we used overall survival for analysis. The Kaplan–Meier curve analysis in patients who received radiotherapy revealed that those who had a high RRPRS-risk score had significantly worse overall survival (OS) compared to those with a low RRPRS-risk score (P = 0.02) (Additional file 1: Fig. S2a). In contrast, no significant difference was observed in the non-radiotherapy group (Additional file 1: Fig. S2b). Additional file 1: Fig. S2c showed the relationship between risk score distribution and nine-genes expression distribution of patients in the CGGA database. In summary, the results suggest that the RRPRS performed well in predicting the progression-free survival (PFS) in patients undergoing radiation therapy.
Stratified PFS analysis based on the risk model in the validation set of the TCGA cohort
To verify the predictive efficiency in stratified cohorts, patients were stratified according to other clinical parameters in the validation set, such as sex, age (≤ 60/> 60 years), and CNS WHO classification (Grade II/III/IV). Kaplan–Meier curves showed that the high-risk group had a shorter PFS than the low-risk group in all subgroups (P ≤ 0.001) (Fig. 5a–f), indicating that the RRPRS risk score is a robust predictor of PFS across all subgroups. We further explored the relationship between the risk score and clinical parameters in patients with glioma. Higher risk scores were associated with higher histopathological glioma grade (Fig. 5g). Additionally, IDH wildtype status and MGMT promoter unmethylated status demonstrated higher risk scores, indicating that the risk score is associated with resistance to chemoradiotherapy. Overall, our results showed that the RRPRS risk score is a reliable predictor of PFS in glioma patients.
Stratified PFS analysis based on the risk model in the validation set of the TCGA cohort. Based on the RRPRS, stratified PFS analysis was performed in patients with glioma with different clinical parameters for the validation set, such as sex (a, b), age group (c, d), CNS WHO classification (e, f), and the relationship between the risk score and histological type (g), IDH mutation status (h), MGMT promoter status (i). Significance for survival analysis was calculated using Cox regression, with the red line representing the RRPRS-high-risk group and the blue line representing the low-risk group
Survival analysis for the genes in the RRPRS
To determine the prognostic value of the nine genes in the RRPRS, we calculated the risk score for each patient with glioblastoma in the TCGA dataset and divided them into high-risk and low-risk groups based on the median risk score. We then performed a Kaplan–Meier survival analysis for each of the nine genes using the “survival and survminer package” in R (Fig. 6a–i). The results showed that these nine genes significantly affected the PFS of the patients, and most of the genes also impacted the OS of patients with GBM (Additional file 1: Fig. S3). The survival time of PFS and OS in the high-expression group was significantly lower than that in the low-expression group. However, there was no significant difference in OS between the high and low-expression groups for NPTN and TMEM71.
Survival analysis associated with the genes in Radiosensitivity-Related Prognostic Risk Signature (RRPRS). Kaplan–Meier plots with the PFS probability of the patients according to stratification by the expression levels of each individual gene. The ordinate axis represents the probability of survival (0–1), and the abscissa axis represents the PFS in days. Blue represents patients with gene expression levels below the median expression of the gene, and red represents patients with expression levels above the median
Establishment of nomograms to predict the OS and PFS in patients with glioma
To further improve the predictive accuracy of the RRPRS, we established prognostic nomograms for PFS and OS, which included the risk score and other independent prognostic factors from the multivariate analysis. As shown in Fig. 7a and c, the higher the total score based on the sum of the assigned numbers for each factor in the nomograms, the worse the 1-year, 2-year, and 3-year PFS and OS rates. Subsequent analysis showed that the 1-, 2-, and 3-year PFS and OS calibration curves were close to the ideal curve, suggesting that the nomogram could accurately predict the radiation therapy outcomes of patients with glioma (Fig. 7b and d).
Establishment of nomograms to predict OS and PFS in patients with glioma. The nomogram a, c covering gender, age, Karnofsky physical status (KPS), IDH mutation status, 1p19q codeletion status and risk score, at 1-year, 2-year, and 3-year in patients with glioma. b, d calibration curve for the PFS and OS nomogram models