Abstract
Objective
This study aims to investigate the mechanism by which biomimetic composite hydrogels loaded with bone marrow mesenchymal stem cells (BMSCs) derived microRNA-19b-3p/WWP1 axis through extracellular vesicles (EVs) affect the new bone formation in rat bone defects.
Methods
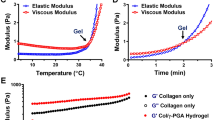
First, synthesize the bionic composite hydrogel Gel-OCS/MBGN. Characterize it through field-emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), and FTIR. Then, conduct performance tests such as rheology, dynamic mechanical analysis, in vitro mineralization, and degradation. Rat BMSCs were selected for in vitro cell experiments, and EVs derived from BMSCs were obtained by differential centrifugation. The EVs were loaded onto Gel-OCS/MBGN to obtain Gel-OCS/MBGN@EVs hydrogel. Cell viability and proliferation were detected by live/dead cell staining and CCK-8 assay, respectively. ALP and ARS staining was used to evaluate the osteogenic differentiation of BMSCs. Differential gene expression analysis of osteogenic differentiation was performed using high-throughput sequencing. TargetScan database predicted the binding site between miR-19b-3p and WWP1, and a dual-luciferase reporter assay was performed to confirm the targeting binding site. A rat bone defect model was established, and new bone formation was evaluated by Micro-CT, H&E staining, and Masson's trichrome staining. Immunofluorescence staining and immunohistochemistry were used to detect the expression levels of osteogenic-related factors in rat BMSCs. RT-qPCR and Western blot were used to detect the expression levels of genes and proteins in tissues and cells.
Result
Gel-OCS/MBGN was successfully constructed and loaded with EVs, resulting in Gel-OCS/MBGN@EVs. The in vitro drug release experiment results show that Gel-OCS/MBGN could sustainably release EVs. Further experiments have shown that Gel-OCS/MBGN@EVs could significantly promote the differentiation of BMSCs into osteoblasts. Experiments have shown that WWP1 is a key factor in osteogenic differentiation and is regulated by miR-19b-3p. EVs promote osteogenic differentiation by suppressing WWP1 expression through the transmission of miR-19b-3p. In vivo animal experiments have demonstrated that Gel-OCS/MBGN@EVs significantly promote bone repair in rats with bone defects by regulating the miR-19b-3p/WWP1 signaling axis.
Conclusion
Functional Gel-OCS/MBGN@EVs were obtained by constructing Gel-OCS/MBGN and loading EVs onto it. EVs could deliver miR-19b-3p to BMSCs, inhibit the expression of WWP1, and promote the osteogenic differentiation of BMSCs, ultimately promoting bone regeneration in rats with bone defects.
Graphical Abstract

Similar content being viewed by others
Introduction
The use of bone transplantation utilizes the principle of "tension-stress law". During the puncture process outside the body, the steel nail is fixed on the bone in the fracture area, and the bone is gradually lengthened by bone cutting [1]. During this process, both elongation and compression regions benefit the formation of calli until the bone defect is healed [2, 3]. Although bone transplantation surgery has the advantages of simple operation, small trauma, and fast bone repair, its surgical operation is complex, and the incidence of complications is high [1, 4].
With the development of bone tissue engineering (BTE), increasing evidence suggests that extracellular vesicles (EVs) derived from mesenchymal stem cells, especially exosomes, have excellent bone regenerative potential [5, 6]. However, current research on the repair of bone defects by MSC-EVs is still in its infancy [5]. Biomaterials with biological activity, such as scaffolds combined with MSCs and their secreted factors EVs, have been widely used in bone defect repair [7]. Research has shown that biomaterials such as hydrogels or scaffolds loaded with mesenchymal stem cells-derived extracellular vesicles could significantly promote osteogenesis and angiogenesis, thereby effectively repairing bone defects [8, 9]. Chondroitin sulfate (CS) is a glycosaminoglycan found in the non-collagen extracellular matrix (ECM) of human bones. Research has shown that CS improves bone regeneration and enhances the arrangement of growth factors involved in bone regeneration. Mesoporous bioactive glass nanoparticles (MBGNs) exhibit excellent biocompatibility, bioactivity, osteogenic, and angiogenic properties. When interacting with polymer matrices and biomolecules, they can enhance mechanical strength and biological activity [10]. Hydrogels can mimic the structure of the extracellular matrix (ECM), providing a 3D environment for cell adhesion, growth, and proliferation. Studies have shown that the biomimetic composite hydrogel Gel-OCS/MBGN has good osteogenic and angiogenic properties. When combined with EV, Gel-OCS/MBGN can improve cell migration, proliferation, and chondrogenic differentiation, as well as promote the formation of glycosaminoglycans, extracellular matrix, and type II collagen [11].
The research by Guanghui Xu et al. showed that EVs derived from HMSC-mediated miR-21 and miR-19b could regulate the apoptosis and differentiation of neurons in spinal cord injury patients [12]. In addition, studies have shown that c-Myc transported by extracellular vesicles derived from tumors promotes the proliferation of bronchial cells in the lungs through miR-19b and miR-92a [13]. Previous studies have demonstrated that miRNAs could regulate cell apoptosis, proliferation, and differentiation processes. SMURF1 targets miR-19b-3p modified bone marrow-derived mesenchymal stem cells composite with PLLA scaffold have been proven to enhance osteogenic activity and repair critical bone defects [5C). GO and KEGG enrichment analysis of candidate target genes revealed their involvement in biological processes such as ossification, positive regulation of peptidyl-tyrosine phosphorylation, bone mineralization, cytokine-cytokine receptor interaction, and rheumatoid arthritis (Fig. 5D, E, Additional file 2: Table S1). Further, obtain key factors involved in osteogenic differentiation through machine learning algorithms. A model was constructed by performing Lasso regression on the 22 differentially expressed genes obtained above, and 4 core genes were identified: CCL5, LEP, CXCL8, and WWP1 (Fig. 6A, B). Among these four genes, only WWP1 was significantly down-regulated in osteoblasts, while CCL5, LEP, and CXCL8 expression was significantly up-regulated in osteoblasts (Fig. 6C). WWP1 is considered an inhibitor of osteoblast differentiation [22]. Therefore, we will select WWP1 for further study, including its upstream miRNA and related mechanisms.
Candidate gene screening for osteogenic differentiation of BMSCs. Note A Heatmap of differential analysis of high-throughput sequencing data (BMSC group, n = 3; osteoblast (OB) group, n = 3); B Volcano plot of differential analysis of high-throughput sequencing data. Red dots represent up-regulated genes, green dots represent down-regulated genes, and black dots represent genes with no significant differential expression (BMSC group, n = 3; osteoblast (OB) group, n = 3); C Venn diagram of the intersection between Genecard database and differentially expressed genes obtained by high-throughput sequencing data analysis; D GO enrichment analysis results of candidate genes, and the horizontal axis represents GeneRatio; E Circos plot of KEGG-GO integrated analysis of candidate genes
Key gene selection for osteogenic differentiation of BMSCs. Note A Lasso coefficient path diagram of differentially expressed genes in RNA-seq; B Cross-validation curve of differentially expressed genes in RNA-seq; C Expression levels of CCL5, LEP, CXCL8, and WWP1 in high-throughput sequencing data of BMSC group (n = 3) and osteoblast (OB) group (n = 3)
EVs may inhibit the expression of WWP1 by delivering miR-19b-3p
RT-qPCR analysis showed that the expression of miR-19b-3p significantly increased while that of WWP1 significantly decreased in BMSCs undergoing osteogenic differentiation (Fig. 7A).
Regulatory role of miR-19b-3p on WWP1. Note A Expression levels of miR-19b-3p and WWP1 in the osteogenic differentiation of BMSCs were detected by RT-qPCR (n = 12 per group, * indicates significantly different from the Sham group with P < 0.05). B TargetScan was used to predict the binding sites of miR-19b-3p and WWP1. C A dual luciferase reporter assay was performed to confirm the targeting relationship between miR-19b-3p and WWP1 (experiments were repeated three times, * indicates a significantly different from the mimic NC group with P < 0.05). D RT-qPCR detected expression levels of miR-19b-3p and WWP1 in cells from each group (experiments were repeated three times, * indicates significantly different from the mimic NC group with P < 0.05, # indicates significantly different from the inhibitor NC group with P < 0.05). E The protein expression levels of WWP1 in cells from each group were detected by Western blot (experiments were repeated three times, * indicates significantly different from the mimic NC group with P < 0.05, # indicates significantly different from the inhibitor NC group with P < 0.05)
To investigate whether miR-19b-3p could target WWP1, the binding site between miR-19b-3p and WWP1 was obtained from the TargetScan database (Fig. 7B). Subsequently, the results of the dual-luciferase reporter gene assay showed that in WWP1-WT, compared with the mimic NC group, there was a significant decrease in luciferase activity in BMSCs cells of the miR-19b-3p mimic group (Fig. 7C), indicating that miR-19b-3p could target and inhibit WWP1.
Further research indicates that miR-19b-3p could regulate the expression of WWP1. The results of RT-qPCR and Western blot detection showed that in the miR-19b-3p mimic group, the expression level of miR-19b-3p in BMSCs cells was significantly increased, while the expression level of WWP1 was significantly decreased; In the miR-19b-3p inhibitor group, the expression level of miR-19b-3p in the cells was significantly decreased, while the expression level of WWP1 was significantly increased (Fig. 7D, E).
In conclusion, miR-19b-3p could regulate osteogenic differentiation by targeting and inhibiting the expression of WWP1.
EVs could transfer miR-19b-3p to suppress WWP1 expression and promote osteogenic differentiation
We first test whether EVs could transmit miR-19b-3p and regulate the expression of WWP1. We labeled BMSCs-EVs with DiO fluorescence and co-cultured them with BMSCs for 24 h. We used confocal fluorescent microscopy to observe, and the results showed that with the prolonged incubation time, many EVs entered the cytoplasm of BMSCs (Fig. 8A). We also used qRT-PCR to detect the silencing effect of miR-19b-3p in BMSCs and detected the expression of miR-19b-3p in both BMSCs and their derived EVs. The results showed that after silencing miR-19b-3p in BMSCs, the expression levels of miR-19b-3p in BMSCs and EVs were significantly decreased. Compared with the BMSCs-inhibitor NC group, the expression level of miR-19b-3p was significantly decreased in the BMSCs-miR-19b-3p inhibitor group (Fig. 8B).
Regulatory effects of miR-19b-3p delivered by EVs derived from BMSCs on the WWP1 axis. Note A BMSCs uptake of EVs at different time points was observed by confocal fluorescence microscopy, scale bar: 25 μm; B The expression of miR-19b-3p in BMSCs and BMSC-derived EVs was detected by RT-qPCR (n = 12 per group, * indicates P < 0.05 compared to the BMSCs-inhibitor NC group); C The gene expression levels of miR-19b-3p and WWP1 were detected by RT-qPCR in various groups of cells (experiment repeated three times, * indicates P < 0.05 compared with the PBS group, # indicates P < 0.05 compared with the EVs-inhibitor NC group); D The protein expression level of WWP1 in each group of cells was detected by Western blot (experiment repeated three times, * indicates P < 0.05 compared with the PBS group, # indicates P < 0.05 compared with the EVs-inhibitor NC group); E The gene expression levels of miR-19b-3p and WWP1 were detected by RT-qPCR in various groups of cells (experiment repeated three times, * indicates P < 0.05 compared to the oe-NC + PBS group, # indicates P < 0.05 compared to the oe-NC + EVs group); F The protein expression level of WWP1 in each group of cells was detected by Western blot (experiment repeated three times, * indicates P < 0.05 compared to the oe-NC + PBS group, # indicates P < 0.05 compared to the oe-NC + EVs group); G ALP staining was used to detect the activity of ALP in various groups of BMSCs, scale bar: 50 μm; H ARS was used to detect osteogenic differentiation of BMSCs in each group, scale bar: 50 μm (experiment repeated three times, * indicates P < 0.05 compared to the oe-NC + PBS group, # indicates P < 0.05 compared to the oe-NC + EVs group); I, J RT-qPCR and Western blot were used to detect the gene and protein expression levels of osteogenic differentiation markers Runx2, Osterix, Alpl, Opn, and Ocn in various groups of BMSCs. The experiment was repeated three times. * indicates P < 0.05 compared with the oe-NC + PBS group, # indicates P < 0.05 compared with the oe-NC + EVs group
Next, we will co-culture BMSCs with or without EVs and incubate EVs extracted from transfected BMSCs with BMSCs cells. We used qRT-PCR and Western blot to detect that the expression level of miR-19b-3p in EVs derived from BMSCs significantly increased after silencing miR-19b-3p, while the expression level of WWP1 significantly decreased. Compared with the PBS group, the expression level of miR-19b-3p in BMSCs was significantly increased, and the expression level of WWP1 was significantly decreased in the EVs group. Compared with the EVs-inhibitor NC group, the expression level of miR-19b-3p in BMSCs was significantly reduced in the EVs-miR-19b-3p inhibitor group, while the expression level of WWP1 was significantly increased (Fig. 8C, D). The above results indicate that EVs derived from BMSCs could target and inhibit the expression of WWP1 by delivering miR-19b-3p into BMSCs.
Then, we further investigated the impact of EVs derived from BMSCs on cell functions by delivering miR-19b-3p to suppress the expression of WWP1. We detected the expression of the osteogenic differentiation markers Run 2, Osterix, Alpl, Opn, and Ocn in BMSCs by qRT-PCR and Western blot. Results showed that compared with the oe-NC + PBS group, the expression of bone differentiation markers significantly increased in the oe-NC + EVs group, decreased significantly in the oe-WWP1 + PBS group, and decreased significantly in the oe-WWP1 + EVs group compared with the oe-NC + EVs group. Meanwhile, the result was confirmed using ALP and ARS staining (Fig. 8E–J).
Therefore, our study demonstrates that EVs derived from BMSCs could promote osteogenic differentiation of BMSCs by regulating WWP1 expression through transferring miR-19b-3p.
Biomimetic composite hydrogel Gel-OCS/MBGN promotes bone defect repair through regulation of the miR-19b-3p/WWP1 axis by loading EVs
We set up a total of four experimental groups: the PBS (blank control) group, Gel-OCS/MBGN group, Gel-OCS/MBGN@EVs-inhibitor NC group, and Gel-OCS/MBGN@EVs-miR-19b-3p inhibitor group. We retrieved the femurs for further investigation at the 8th-week post-implantation of hydrogel in a rat model. According to the results shown in Fig. 9A, Gel-OCS/MBGN group covered the defect area with newly regenerated tissue compared to the PBS group. The junction of the defect showed almost completely matured bone tissue with complete and continuous bone closure. Gel-OCS/MBGN@EVs-inhibitor NC group showed complete and smooth regeneration of new tissue, which was well integrated with the surrounding primitive cartilage, forming a morphology similar to that of normal cartilage. Compared with the Gel-OCS/MBGN@EVs-inhibitor NC group, a small amount of regenerative new bone tissue and less bone formation were observed on the bone surface in the Gel-OCS/MBGN@EVs-miR-19b-3p inhibitor group. The surface of the newly-formed tissue was rough, and there was no complete and continuous bone closure, which could not be combined with cartilage.
Bionic composite hydrogel Gel-OCS/MBGN regulates bone defect healing through loading EVs to modulate the miR-19b-3p/WWP1 axis. Note A Observing the healing of femurs in each group of rats by naked eye; B, C Observing the new bone formation at the site of femoral defect in each group of rats by X-ray and Micro-CT; D, E BV/TV and BMC analysis in (C) (* indicates P < 0.05 compared with PBS group, # indicates P < 0.05 compared with Gel-OCS/MBGN@EVs-inhibitor NC group); F Pathological changes in new bone formation at the site of femoral defect in each group of rats detected by H&E staining, Scale bar: 200 μm; G Collagen fiber formation in new bone formation at the site of femoral defect in each group of rats detected by Masson staining, Scale bar: 100 μm; H, I Expression of OPN, a marker of osteogenic differentiation, detected by immunohistochemical staining in each group of rats at the site of femoral defect (cytoplasm), Scale bar: 50 μm, red arrows indicate OPN-positive cells; J Expression levels of miR-19b-3p and WWP1 at the site of femoral defect in each group of rats detected by RT-qPCR (N = 6, * indicates P < 0.05 compared with PBS group, # indicates P < 0.05 compared with Gel-OCS/MBGN@EVs-inhibitor NC group). K Western blot was used to detect the protein expression levels of WWP1 in the femoral defect of each group (N = 6, * indicates P < 0.05 compared with the PBS group, # indicates P < 0.05 compared with the Gel-OCS/MBGN@EVs-inhibitor NC group)
Meanwhile, the X-ray results showed that the bone defects in the PBS group remained relatively large, while in comparison, the bone pores in the Gel-OCS/MBGN rats were significantly reduced, and their bone defects had almost fully healed. Additionally, a remodeling process was observed, indicating accelerated bone repair. Compared with the Gel-OCS/MBGN@EVs-inhibitor NC group, the healing of bone defects in rats was reduced, the speed of resha** and repairing was decreased, the bone porosity in rats was increased, and a small amount of new autologous bone tissue was formed around the bone defect site in the Gel-OCS/MBGN@EVs-miR-19b-3p inhibitor group (Fig. 9B).
Through Micro-CT imaging, we observed the formation of new bone tissue at the bone defect site in rats. We found that only a small amount of new bone was formed in the PBS group rats at the defect site. In comparison, the Gel-OCS/MBGN group rats had increased bone volume, number of trabeculae, and bone formation rate, indicating a significant increase in new bone formation. Compared with Gel-OCS/MBGN@EVs-inhibitor NC, the Gel-OCS/MBGN@EVs-miR-19b-3p inhibitor group showed limited new bone formation. The number of trabeculae and the bone formation rate increase or decrease, and the bone structure and density increase or decrease (Fig. 9C).
Quantitative analyses of bone volume fraction (BV/TV) and bone mineral content (BMC) showed that compared with the PBS group, BV/TV and BMC were significantly increased in the Gel-OCS/MBGN group, indicating a significant increase in bone quantity and mineral content. Compared with the Gel-OCS/MBGN@EVs-inhibitor NC group, BV/TV and BMC were significantly decreased in the Gel-OCS/MBGN@EVs-miR-19b-3p inhibitor group, indicating a decrease in bone regeneration ability and a significant reduction in bone trabeculae, bone quantity, and mineral content, as well as a decrease in the ability to heal bone defects (Fig. 9D–G).
To confirm the role of Gel-OCS/MBGN@EVs in regulating osteogenic differentiation, we used immunohistochemistry to detect the expression of markers. Results showed that compared with the PBS group, there was a significant increase in the expression of OPN, a bone differentiation marker in Gel-OCS/MBGN. Moreover, compared with Gel-OCS/MBGN@EVs-inhibitor NC group, OPN expression at the boundaries between fibrous tissues and bone tissues was significantly reduced in Gel-OCS/MBGN@EVs-miR-19b-3p inhibitor group (Fig. 9H-I). Further RT-qPCR detection revealed that compared to the PBS group, the expression level of miR-19b-3p was significantly increased, while the expression level of WWP1 was significantly decreased in the BMSCs of the Gel-OCS/MBGN group. Compared with Gel-OCS/MBGN@EVs-inhibitor NC group, the expression level of miR-19b-3p in BMSCs was significantly decreased, while the expression level of WWP1 was significantly increased in Gel-OCS/MBGN@EVs-miR-19b-3p inhibitor group (Fig. 9J). By Western blotting to detect the expression of WWP1, we found that compared with the PBS group, the WWP1 expression was significantly decreased in the Gel-OCS/MBGN group, while compared with the Gel-OCS/MBGN@EVs-inhibitor NC group, the WWP1 expression was significantly increased in the Gel-OCS/MBGN@EVs-miR-19b-3p inhibitor group (Fig. 9K, M).
Our research findings suggest that Gel-OCS/MBGN@EVs may promote bone regeneration in femoral defect rats by inhibiting WWP1 expression via delivering miR-19b-3p to facilitate osteogenic differentiation.
Discussion
We successfully constructed a composite hydrogel GEL-OCS/MBGN and loaded EVs into it. Previous research reports have shown that using compound hydrogels as carriers could enhance EVs' stability and protective effect [23]. Moreover, combining nanomaterials with EVs could enhance the drug-delivery ability of EVs [24]. Using OCS and MBGN as materials could enhance the biocompatibility and biodegradability of the materials [25].
Mesenchymal stem cells (hBMSCs) and extracellular vesicles (EVs) in the bone marrow are potentially valuable in promoting bone regeneration. In these studies, EVs from different sources were used to promote the osteogenic differentiation of hBMSCs. For instance, EVs derived from skeletal muscle cells could promote osteogenic differentiation of hBMSCs by activating specific signaling pathways [26]. Another study showed that loading EVs derived from dental pulp stem cells into hydroxyapatite/polylactide composite materials could promote bone regeneration [27]. These research results are consistent with the findings of our study, which indicate that loading EVs into biomimetic composite hydrogel GEL-OCS/MBGN could promote rat bone defect repair by regulating the miR-19b/WWP1 axis. This result suggests that combining biologically active EVs with biocompatible carrier materials could synergistically promote bone regeneration. In addition, research on combining GEL-OCS/MBGN material with hydroxyapatite has also demonstrated that this composite material has higher biological activity and biocompatibility, further enhancing its potential applications in bone regeneration [10]. These studies provide valuable insights for future clinical applications in bone defect repair and skeletal regeneration.
During the process of osteogenesis, bone marrow mesenchymal stem cells (BMSCs) gradually differentiate into osteoblasts and secrete collagen fibers around the osteoblasts. This promotes the deposition of calcium on the collagen fibers, resulting in further transformation into osteocytes [28]. Studies have shown that miR-19b-3p enhances osteogenic activity and its high expression is associated with osteogenic differentiation [48].
Constructing rat models with femoral bone defects
We purchased 24 SD male rats (7 weeks old, weighing 250–300 g) with strain code 101 from Bei**g Vital River Laboratory Animal Technology Co., Ltd. These rats were raised in an SPF-grade animal laboratory with a humidity of 60% to 65%, a temperature of 22–25 °C, and free access to food and water under a 12-h light–dark cycle. After one week of adaptive feeding, we observed the health conditions of the rats before starting the experiment. This experiment has been approved by the Animal Ethics Committee of The Third Hospital of Hebei Medical University and conforms to the principles for the management and use of experimental animals in the local area.
When performing the femoral defect surgery, we performed general anesthesia on each rat. We made a 1.5 cm longitudinal incision in the center of the palpable bone protrusion on the outer side of the femoral condyle of each leg while taking sufficient sterile precautions. Then we carefully dissected the subcutaneous tissue, fascia, muscles, and periosteum, exposing the underlying bone. We induce bone defects using an oral micromotor to form a 4 mm deep hole in the subchondral bone below with a 4 mm annular bone drill. Next, we will implant corresponding hydrogels into the defect area, followed by exposure to 405 nm light for 90 s (25 mW/cm2) to form a secondary network. Finally, we carefully layered and sutured the soft tissues and skin. After 8 weeks, we euthanized rats to obtain femurs. We fixed it using over 4% formaldehyde solution (24 h) and preserved it in 75% ethanol for subsequent analysis [38, 49, 50].
We randomly divided the rats into 4 groups, with 6 rats in each group: (1) PBS group; (2) Gel-OCS/MBGNs@EVs group (injection of Gel-OCS/MBGN@EVs hydrogel); (3) Gel-OCS/MBGNs@EVs-inhibitor NC group (transfecting inhibitor NC obtained EVs into BMSCs, and Gel-OCS/MBGNs@EVs-inhibitor NC hydrogel was prepared for injection); (4) Gel-OCS/MBGNs@EVs-miR-19b-3p inhibitor group (EVs were obtained by transfecting miR-19b-3p inhibitor into BMSCs, and Gel-OCS/MBGNs@EVs-miR-19b-3p inhibitor hydrogel was prepared for injection). Inject 100 μL water gel per group, and the final concentration of EVs is 0.2 μg/μL.
Micro-CT and X-ray
Bruker's Micro-CT used a source current of 280 µA, a source voltage of 90 kV, and an exposure time of 550 ms. Each defect region was scanned and analyzed in sagittal and axial planes with the same calibration parameters and reconstructed using NRecon software. To quantify the newly mineralized tissue, bone mineral content (BMC) and new bone volume (BV/TV%) were calculated. After the specimen was taken, X-ray images were also taken [38, 50, 51].
H&E staining and Masson staining
After decalcification in ethylenediaminetetraacetic acid (EDTA) for 6 weeks, the femur tissue was dehydrated and embedded in paraffin through a graded ethanol series. After processing, cut it into 6 μm thick slices for staining. According to the instructions, use Su Mu **g & Yi Hong (H&E, G1076-500ML, Servicebio) and Masson's trichrome staining kit (G1340, Solarbio, Bei**g, China) to stain the slices [38, 39, 52].
Statistical analysis
Data statistical analysis required for this study was conducted using the SPSS 21.0 software produced by IBM. The mean ± standard deviation represents the measurement data. Firstly, normality and homoscedasticity tests are conducted. Suppose the data fit the normal distribution and has equal variances. In that case, the t-test is used to compare two groups of data, and a one-way analysis of variance is used to compare multiple data groups. Tukey's method is used for post-hoc tests. When comparing different data groups at different time points, repeated measures analysis of variance is adopted, and Tukey's method is used for the post-hoc test. When the P value is less than 0.05, it indicates that the difference is statistically significant.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Huang W, Zhu W, Lu W. Comparison of iliac bone transplantation with bone transport in the treatment of femur fracture and bone defect [retracted in: Evid Based Complement Alternat Med. 2023 Jun 21;2023:9820361]. Evid Based Complement Alternat Med. 2022;2022:5358923. https://doi.org/10.1155/2022/5358923.
Ferner F, Lutter C, Dickschas J. Retrograder Femursegmenttransportnagel – eine Anwendung bei posttraumatischem Knochendefekt mit Komplexdeformität [Retrograde bone transport nail in a posttraumatic femoral bone defect]. Unfallchirurg. 2021;124(5):412–8. https://doi.org/10.1007/s00113-020-00916-1.
Zhang S, Wang H, Zhao J, Xu P, Shi H, Mu W. Treatment of post-traumatic chronic osteomyelitis of lower limbs by bone transport technique using mono-lateral external fixator: Follow-up study of 18 cases. J Orthop Sci. 2016;21(4):493–9. https://doi.org/10.1016/j.jos.2016.04.010.
Wei J, Lu M, Zhao L, Zeng X, He L. Free bone grafting improves clinical outcomes in anterior shoulder instability with bone defect: a systematic review and meta-analysis of studies with a minimum of 1-year follow-up. J Shoulder Elbow Surg. 2022;31(4):e190–208. https://doi.org/10.1016/j.jse.2021.10.023.
Wang D, Cao H, Hua W, et al. Mesenchymal stem cell-derived extracellular vesicles for bone defect repair. Membranes (Basel). 2022;12(7):716. https://doi.org/10.3390/membranes12070716.
Zhou X, Cao H, Guo J, Yuan Y, Ni G. Effects of BMSC-derived EVs on bone metabolism. Pharmaceutics. 2022;14(5):1012. https://doi.org/10.3390/pharmaceutics14051012.
Wu D, Chang X, Tian J, et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis [published correction appears in J Nanobiotechnology. 2023 Jul 10;21(1):217]. J Nanobiotechnol. 2021;19(1):209. https://doi.org/10.1186/s12951-021-00958-6.
Liang W, Han B, Hai Y, Sun D, Yin P. Mechanism of action of mesenchymal stem cell-derived exosomes in the intervertebral disc degeneration treatment and bone repair and regeneration. Front Cell Dev Biol. 2022;9:833840. https://doi.org/10.3389/fcell.2021.833840.
Huang CC, Kang M, Shirazi S, et al. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 2021;126:199–210. https://doi.org/10.1016/j.actbio.2021.03.030.
Zhou L, Fan L, Zhang FM, et al. Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioact Mater. 2020;6(3):890–904. https://doi.org/10.1016/j.bioactmat.2020.09.012.
Hu H, Dong L, Bu Z, et al. miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. J Extracell Vesicles. 2020;9(1):1778883. https://doi.org/10.1080/20013078.2020.1778883.
Xu G, Ao R, Zhi Z, Jia J, Yu B. miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J Cell Physiol. 2019;234(7):10205–17. https://doi.org/10.1002/jcp.27690.
Borzi C, Calzolari L, Ferretti AM, et al. c-Myc shuttled by tumour-derived extracellular vesicles promotes lung bronchial cell proliferation through miR-19b and miR-92a. Cell Death Dis. 2019;10(10):759. https://doi.org/10.1038/s41419-019-2003-5.
**ong A, He Y, Gao L, et al. Smurf1-targeting miR-19b-3p-modified BMSCs combined PLLA composite scaffold to enhance osteogenic activity and treat critical-sized bone defects. Biomater Sci. 2020;8(21):6069–81. https://doi.org/10.1039/d0bm01251c.
**aoling G, Shuaibin L, Kailu L. MicroRNA-19b-3p promotes cell proliferation and osteogenic differentiation of BMSCs by interacting with lncRNA H19. BMC Med Genet. 2020;21(1):11. https://doi.org/10.1186/s12881-020-0948-y.
Liu D, Wang B, Qiu M, Huang Y. MiR-19b-3p accelerates bone loss after spinal cord injury by suppressing osteogenesis via regulating PTEN/Akt/mTOR signalling. J Cell Mol Med. 2021;25(2):990–1000. https://doi.org/10.1111/jcmm.16159.
Zhu Y, Long HT, Zeng L, et al. MiR-19b-3p regulates osteogenic differentiation of PDGFRα+ muscle cells by specifically targeting PTEN. Cell Biol Int. 2019;43(5):565–73. https://doi.org/10.1002/cbin.11133.
Wang X, Omar O, Vazirisani F, Thomsen P, Ekström K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE. 2018;13(2):e0193059. https://doi.org/10.1371/journal.pone.0193059.
Huang Y, Xu Y, Feng S, He P, Sheng B, Ni J. miR-19b enhances osteogenic differentiation of mesenchymal stem cells and promotes fracture healing through the WWP1/Smurf2-mediated KLF5/β-catenin signaling pathway. Exp Mol Med. 2021;53(5):973–85. https://doi.org/10.1038/s12276-021-00631-w.
Li L, Zhang Y, Mu J, et al. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20(6):4298–305. https://doi.org/10.1021/acs.nanolett.0c00929.
Hu J, Li X, Chen Y, et al. The protective effect of WKYMVm peptide on inflammatory osteolysis through regulating NF-κB and CD9/gp130/STAT3 signalling pathway. J Cell Mol Med. 2020;24(2):1893–905. https://doi.org/10.1111/jcmm.14885.
Zhao L, Huang J, Zhang H, et al. Tumor necrosis factor inhibits mesenchymal stem cell differentiation into osteoblasts via the ubiquitin E3 ligase Wwp1. Stem Cells. 2011;29(10):1601–10. https://doi.org/10.1002/stem.703.
Hu X, Wang Y, Zhang L, Xu M. Formation of self-assembled polyelectrolyte complex hydrogel derived from salecan and chitosan for sustained release of Vitamin C. Carbohydr Polym. 2020;234:115920. https://doi.org/10.1016/j.carbpol.2020.115920.
Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585–98. https://doi.org/10.1080/10717544.2020.1748758.
Qamar SA, Riasat A, Jahangeer M, et al. Prospects of microbial polysaccharides-based hybrid constructs for biomimicking applications. J Basic Microbiol. 2022;62(11):1319–36. https://doi.org/10.1002/jobm.202100596.
**g H, He X, Zheng J. Exosomes and regenerative medicine: state of the art and perspectives. Transl Res. 2018;196:1–16. https://doi.org/10.1016/j.trsl.2018.01.005.
Ghandforoushan P, Hanaee J, Aghazadeh Z, et al. Enhancing the function of PLGA-collagen scaffold by incorporating TGF-β1-loaded PLGA-PEG-PLGA nanoparticles for cartilage tissue engineering using human dental pulp stem cells. Drug Deliv Transl Res. 2022;12(12):2960–78. https://doi.org/10.1007/s13346-022-01161-2.
Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–9. https://doi.org/10.2215/CJN.04151206.
Li X, Wang J, Wu C, Lu X, Huang J. MicroRNAs involved in the TGF-β signaling pathway in atherosclerosis. Biomed Pharmacother. 2022;146:112499. https://doi.org/10.1016/j.biopha.2021.112499.
Zhang Y, **e RL, Croce CM, et al. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci U S A. 2011;108(24):9863–8. https://doi.org/10.1073/pnas.1018493108.
**e P, Kondeti VK, Lin S, Haruna Y, Raparia K, Kanwar YS. Withdrawal: Role of extracellular matrix renal tubulo-interstitial nephritis antigen (TINag) in cell survival utilizing integrin αvβ3/focal adhesion kinase (FAK)/phosphatidylinositol 3-kinase (PI3K)/protein kinase B-serine/threonine kinase (AKT) signaling pathway [retraction of: J Biol Chem. 2011 Sep 30;286(39):34131-46]. J Biol Chem. 2019;294(26):10379. https://doi.org/10.1074/jbc.W119.009585.
Hertel FC, Silva ASD, Sabino AP, Valente FL, Reis ECC. Preconditioning methods to improve mesenchymal stromal cell-derived extracellular vesicles in bone regeneration—a systematic review. Biology (Basel). 2022;11(5):733. https://doi.org/10.3390/biology11050733.
** J, Ou Q, Wang Z, et al. BMSC-derived extracellular vesicles intervened the pathogenic changes of scleroderma in mice through miRNAs. Stem Cell Res Ther. 2021;12(1):327. https://doi.org/10.1186/s13287-021-02400-y.
Lou Z, Peng Z, Wang B, Li X, Li X, Zhang X. miR-142-5p promotes the osteoclast differentiation of bone marrow-derived macrophages via PTEN/PI3K/AKT/FoxO1 pathway. J Bone Miner Metab. 2019;37(5):815–24. https://doi.org/10.1007/s00774-019-00997-y.
Chen H, Yang S, Shao R. Long non-coding XIST raises methylation of TIMP-3 promoter to regulate collagen degradation in osteoarthritic chondrocytes after tibial plateau fracture. Arthritis Res Ther. 2019;21(1):271. https://doi.org/10.1186/s13075-019-2033-5.
Huang Y, Liu W, He B, et al. Exosomes derived from bone marrow mesenchymal stem cells promote osteosarcoma development by activating oncogenic autophagy. J Bone Oncol. 2020;21:100280. https://doi.org/10.1016/j.jbo.2020.100280.
Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomed. 2020;15:5911–26. https://doi.org/10.2147/IJN.S249129.
Li R, Zhou C, Chen J, et al. Synergistic osteogenic and angiogenic effects of KP and QK peptides incorporated with an injectable and self-healing hydrogel for efficient bone regeneration. Bioact Mater. 2022;18:267–83. https://doi.org/10.1016/j.bioactmat.2022.02.011.
Xu Y, Zhou J, Liu C, et al. Understanding the role of tissue-specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials. 2021;268:120596. https://doi.org/10.1016/j.biomaterials.2020.120596.
Dalhat MH, Mohammed MRS, Alkhatabi HA, et al. NAT10: an RNA cytidine transferase regulates fatty acid metabolism in cancer cells. Clin Transl Med. 2022;12(9):e1045. https://doi.org/10.1002/ctm2.1045.
Peculis R, Rovite V, Megnis K, et al. Whole exome sequencing reveals novel risk genes of pituitary neuroendocrine tumors. PLoS ONE. 2022;17(8):e0265306. https://doi.org/10.1371/journal.pone.0265306.
Wan J, Liu B. Construction of lncRNA-related ceRNA regulatory network in diabetic subdermal endothelial cells. Bioengineered. 2021;12(1):2592–602. https://doi.org/10.1080/21655979.2021.1936892.
Zhao Y, Wang L, Wu Y, Lu Z, Zhang S. Genome-wide study of key genes and scoring system as potential noninvasive biomarkers for detection of suicide behavior in major depression disorder. Bioengineered. 2020;11(1):1189–96. https://doi.org/10.1080/21655979.2020.1831349.
Li T, Wang W, Gan W, et al. Comprehensive bioinformatics analysis identifies LAPTM5 as a potential blood biomarker for hypertensive patients with left ventricular hypertrophy. Aging (Albany NY). 2022;14(3):1508–28. https://doi.org/10.18632/aging.203894.
Li J, Liu C, Chen Y, et al. Tumor characterization in breast cancer identifies immune-relevant gene signatures associated with prognosis. Front Genet. 2019;10:1119. https://doi.org/10.3389/fgene.2019.01119.
** Z, Ren J, Qi S. Exosomal miR-9-5p secreted by bone marrow-derived mesenchymal stem cells alleviates osteoarthritis by inhibiting syndecan-1. Cell Tissue Res. 2020;381(1):99–114. https://doi.org/10.1007/s00441-020-03193-x.
Lange T, Stracke S, Rettig R, et al. Identification of miR-16 as an endogenous reference gene for the normalization of urinary exosomal miRNA expression data from CKD patients. PLoS ONE. 2017;12(8):e0183435. https://doi.org/10.1371/journal.pone.0183435.
Wu Q, Yi X. Down-regulation of long noncoding RNA MALAT1 protects hippocampal neurons against excessive autophagy and apoptosis via the PI3K/Akt signaling pathway in rats with epilepsy. J Mol Neurosci. 2018;65(2):234–45. https://doi.org/10.1007/s12031-018-1093-3.
Yi G, Zhang S, Ma Y, et al. Matrix vesicles from dental follicle cells improve alveolar bone regeneration via activation of the PLC/PKC/MAPK pathway. Stem Cell Res Ther. 2022;13(1):41. https://doi.org/10.1186/s13287-022-02721-6.
Wang L, Wang J, Zhou X, et al. A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front Bioeng Biotechnol. 2020;8:564731. https://doi.org/10.3389/fbioe.2020.564731.
Ou Q, Miao Y, Yang F, Lin X, Zhang LM, Wang Y. Zein/gelatin/nanohydroxyapatite nanofibrous scaffolds are biocompatible and promote osteogenic differentiation of human periodontal ligament stem cells. Biomater Sci. 2019;7(5):1973–83. https://doi.org/10.1039/c8bm01653d.
Irrera N, Arcoraci V, Mannino F, et al. Activation of A2A receptor by PDRN reduces neuronal damage and stimulates WNT/β-CATENIN driven neurogenesis in spinal cord injury [published correction appears in Front Pharmacol. 2022 Nov 03;13:1073726]. Front Pharmacol. 2018;9:506. https://doi.org/10.3389/fphar.2018.00506.
Acknowledgements
Not applicable.
Funding
This study was supported by Key Science and Technology Research Program of Hebei Provincial Health Commission (20210120), Key Science and Technology Research Program of Hebei Provincial Health Commission (20221202), Funding for Geriatric Disease Prevention and Control of Hebei Provincial Department of Finance and 2022 Government-funded Clinical Medicine Excellence Training Program Leader Project.
Author information
Authors and Affiliations
Contributions
RG conceived and designed research. CW performed experiments. FL interpreted results of experiments. TD analyzed data. TZ prepared figures. RG drafted paper. CW and FL edited and revised manuscript. All authors read and approved final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This experiment has been approved by the Animal Ethics Committee of The Third Hospital of Hebei Medical University and conforms to the principles for the management and use of experimental animals in the local area.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
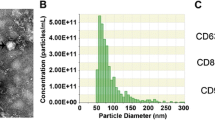
Characterization of BMSCs and EVs. Note: (A) Observe the morphology of BMSCs with an inverted microscope, scale bar = 100 μm; (B) Detect the osteogenic, adipogenic, and chondrogenic differentiation ability of BMSCs by staining with Alizarin Red, Oil Red O, and Alcian Blue, scale bar = 100 μm / 50 μm; (C) Detect the expression of BMSCs markers by flow cytometry; (D) Observe the morphology characteristics of EVs with TEM image, scale bar = 200 μm; (E) Detect the size of EVs by NTA; (F) Detect the expression of EVs markers by Western blot. The experiment should be repeated at least three times.
Additional file 2.
Primer sequences of RT-qPCR, Top 10 KEGG enrichment analysis entries.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, R., Wu, C., Liu, F. et al. Biomimetic composite hydrogel promotes new bone formation in rat bone defects through regulation of miR-19b-3p/WWP1 axis by loaded extracellular vesicles. J Nanobiotechnol 21, 459 (2023). https://doi.org/10.1186/s12951-023-02201-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-023-02201-w