Abstract
Background
Exposure to noxious particles, including cigarette smoke and fine particulate matter (PM2.5), is a risk factor for chronic obstructive pulmonary disease (COPD) and promotes inflammation and cell death in the lungs. We investigated the combined effects of cigarette smoking and PM2.5 exposure in patients with COPD, mice, and human bronchial epithelial cells.
Methods
The relationship between PM2.5 exposure and clinical parameters was investigated in patients with COPD based on smoking status. Alveolar destruction, inflammatory cell infiltration, and pro-inflammatory cytokines were monitored in the smoking-exposed emphysema mouse model. To investigate the mechanisms, cell viability and death and pyroptosis-related changes in BEAS-2B cells were assessed following the exposure to cigarette smoke extract (CSE) and PM2.5.
Results
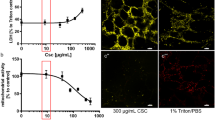
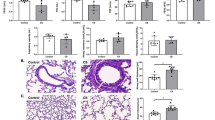
High levels of ambient PM2.5 were more strongly associated with high Saint George’s respiratory questionnaire specific for COPD (SGRQ-C) scores in currently smoking patients with COPD. Combined exposure to cigarette smoke and PM2.5 increased mean linear intercept and TUNEL-positive cells in lung tissue, which was associated with increased inflammatory cell infiltration and inflammatory cytokine release in mice. Exposure to a combination of CSE and PM2.5 reduced cell viability and upregulated NLRP3, caspase-1, IL-1β, and IL-18 transcription in BEAS-2B cells. NLRP3 silencing with siRNA reduced pyroptosis and restored cell viability.
Conclusions
PM2.5 aggravates smoking-induced airway inflammation and cell death via pyroptosis. Clinically, PM2.5 deteriorates quality of life and may worsen prognosis in currently smoking patients with COPD.
Graphical Abstract

Similar content being viewed by others
Background
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease usually caused by prolonged exposure to noxious gases or particles [1]. Although cigarette smoking is an important risk factor in COPD, many patients with COPD are never-smokers [2,3,4]. Occupational exposure and biomass fuels are well-known risk factors in never-smoker COPD [5, 6]. Recent studies have linked particulate matter of diameter ≤ 2.5 μm (PM2.5) to decreased lung function, airway inflammation, and emphysematous changes in the lungs, leading to the development of COPD and increased mortality [7,8,9,10].
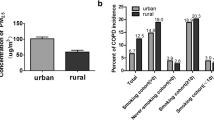
Both PM2.5 and smoking have been reported to promote inflammation and cell death in the lungs [11,12,13,14,15,16]. Particularly, PM2.5 is known to induce various types of cell deaths, including autophagy, necrosis, apoptosis, pyroptosis, and ferroptosis [17]. Recently, pyroptosis has been identified as a crucial process in lung injury. Pyroptosis is an inflammatory type of programmed cell death mediated by caspase-1 and activated by the inflammasome [18, 19]. The inflammasome is an intracellular multi-protein component composed primarily of nucleotide-binding oligomerization domain-like receptor (NLR) family and the pyrin and hematopoietic interferon-inducible nuclear domain protein family [20]. NLR protein-3 (NLRP3) is an important member of NLR family that recognizes and is activated by pathogen-associated molecular patterns or damage-associated molecular patterns [21, 67]. Exposure to PM2.5 was associated with systemic inflammation in patients with COPD [68], and systemic inflammation in COPD was associated with poor QOL [69, 70]. In pyroptosis, the plasma-membrane rapidly ruptures and proinflammatory intracellular contents are released, resulting in pathological inflammation [19]. Moreover, patients with stable COPD had significantly higher plasma IL-1β levels and upregulated expression of the IL1B, NLRP3, and CASP1 genes compared with that in healthy controls [71]. In our investigation, high ambient PM2.5 concentrations were linked to high SGRQ-C scores in currently smoking patients with COPD. Moreover, high SGRQ-C scores were associated with rapid lung function decline and frequent exacerbation [72,73,74]. We can conclude from this study that PM2.5 exposure aggravates smoking-induced airway inflammation and deteriorates QOL of patients with COPD, with local or systemic pyroptosis-mediated inflammation playing an important role. Furthermore, PM2.5 exposure may induce lung function decline and exacerbation in currently smoking patients with COPD.
There are some limitations in our study. First, PM2.5 exposure alone did not alter the total number or the differential proportions of cells in the BALF, nor did it induce peribronchial inflammatory cell infiltration. Second, a 1-week time interval between the last exposure to PM2.5 and euthanasia of the mice may allow clearance of PM2.5 by macrophages. Third, the synergistic effect of PM2.5 and cigarette smoke exposure was prominent in the protein composition of BALF, whereas this synergy was relatively less evident in qPCR levels of lung homogenate. Since both PM2.5 and cigarette smoke were delivered intratracheally, their impact on the alveolar space appears to be more pronounced. Additionally, the alterations observed in protein levels holds greater significance compared with those observed in mRNA. Fourth, although exposure to smoking and PM2.5 caused lung injury and cell death in our experiments, other cell death mechanisms, such as apoptosis, might be involved. Meanwhile, our results demonstrate a significant upregulation of pyroptosis-related genes and proteins, as well as restored cell viability with caspase-1 inhibition and NLRP3 silencing. Fifth, in clinical data, a larger sample size and longer study duration might show a more consistent difference in associations between current and ex-smokers. However, the correlation between SGRQ-C score and PM2.5 concentration in current smokers implies that the combined exposure to smoking and PM2.5 has an additive aggravating effect, and pyroptosis-induced systematic inflammation may be involved.
Conclusions
In conclusion, the combined exposure to PM2.5 and cigarette smoking aggravates smoking-induced airway inflammation and cell death with pyroptosis being one of the dominant mechanisms. In patients with COPD, PM2.5 aggravates the QOL caused by concurrent smoking and may deteriorate lung function and induce exacerbation. COPD is a preventable disease caused by exposure to noxious particles with various synergistic effects. Identifying these addictive effects will contribute to our understanding of the pathogenesis of COPD and the development of effective treatment options.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BALF:
-
Bronchoalveolar lavage fluid
- COPD:
-
Chronic obstructive pulmonary disease
- CSE:
-
Cigarette smoke extract
- DMSO:
-
Dimethyl sulfoxide
- ELISA:
-
Enzyme-linked immunosorbent assay
- GADPH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- H&E:
-
Hematoxylin and eosin
- IFN:
-
Interferon, IL: Interleukin
- LDH:
-
Lactate dehydrogenase
- MLI:
-
Mean linear intercept
- MTT:
-
3-[4,5-imethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide
- NLR:
-
Nucleotide-binding oligomerization domain-like receptor
- NLRP3:
-
NLR protein-3
- PBS:
-
Phosphate-buffered saline
- PM2.5 :
-
Particulate matter of diameter ≤ 2.5 μm
- PMSF:
-
Phenylmethylsulfonyl fluoride
- QOL:
-
Quality of life
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- SGRQ-C:
-
Saint George’s respiratory questionnaire specific for COPD
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
References
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, et al. Global strategy for the diagnosis, management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195:557–82.
Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, Studnicka M, Bateman E, Anto JM, Burney P, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–63.
Tan WC, Sin DD, Bourbeau J, Hernandez P, Chapman KR, Cowie R, FitzGerald JM, Marciniuk DD, Maltais F, Buist AS, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax. 2015;70:822–9.
Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–43.
Paulin LM, Diette GB, Blanc PD, Putcha N, Eisner MD, Kanner RE, Belli AJ, Christenson S, Tashkin DP, Han M, et al. Occupational exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:557–65.
Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187:721–7.
Liu S, Zhou Y, Liu S, Chen X, Zou W, Zhao D, Li X, Pu J, Huang L, Chen J, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax. 2017;72:788–95.
Lamichhane DK, Leem JH, Kim HC. Associations between Ambient Particulate Matter and Nitrogen Dioxide and Chronic Obstructive Pulmonary diseases in adults and effect modification by demographic and lifestyle factors. Int J Environ Res Public Health 2018, 15.
Dai L, Zanobetti A, Koutrakis P, Schwartz JD. Associations of fine particulate matter species with mortality in the United States: a multicity time-series analysis. Environ Health Perspect. 2014;122:837–42.
Doiron D, de Hoogh K, Probst-Hensch N, Fortier I, Cai Y, De Matteis S, Hansell AL. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J 2019, 54.
Xue H, Li MX. MicroRNA-150 protects against cigarette smoke-induced lung inflammation and airway epithelial cell apoptosis through repressing p53: MicroRNA-150 in CS-induced lung inflammation. Hum Exp Toxicol. 2018;37:920–8.
Pan X, Xu K, Li Y, Wang X, Peng X, Li M, Li Y. Interleukin-35 expression protects against cigarette smoke-induced lung inflammation in mice. Biomed Pharmacother. 2019;110:727–32.
Li C, Chen J, Yuan W, Zhang W, Chen H, Tan H. Preventive effect of ursolic acid derivative on particulate matter 2.5-induced chronic obstructive pulmonary disease involves suppression of lung inflammation. IUBMB Life. 2020;72:632–40.
Ogino K, Nagaoka K, Okuda T, Oka A, Kubo M, Eguchi E, Fujikura Y. PM2.5-induced airway inflammation and hyperresponsiveness in NC/Nga mice. Environ Toxicol. 2017;32:1047–54.
Zheng R, Tao L, Jian H, Chang Y, Cheng Y, Feng Y, Zhang H. NLRP3 inflammasome activation and lung fibrosis caused by airborne fine particulate matter. Ecotoxicol Environ Saf. 2018;163:612–9.
Vij N, Chandramani-Shivalingappa P, Van Westphal C, Hole R, Bodas M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am J Physiol Cell Physiol. 2018;314:C73–87.
Wang Y, Zhong Y, Liao J, Wang G. PM2.5-related cell death patterns. Int J Med Sci. 2021;18:1024–9.
Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–14.
Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109.
Rathinam VA, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and Effector functions. Cell. 2016;165:792–800.
Barbe F, Douglas T, Saleh M. Advances in nod-like receptors (NLR) biology. Cytokine Growth Factor Rev. 2014;25:681–97.
Latz E, **ao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411.
Nachmias N, Langier S, Brzezinski RY, Siterman M, Stark M, Etkin S, Avriel A, Schwarz Y, Shenhar-Tsarfaty S, Bar-Shai A. NLRP3 inflammasome activity is upregulated in an in-vitro model of COPD exacerbation. PLoS ONE. 2019;14:e0214622.
Zhang MY, Jiang YX, Yang YC, Liu JY, Huo C, Ji XL, Qu YQ. Cigarette smoke extract induces pyroptosis in human bronchial epithelial cells through the ROS/NLRP3/caspase-1 pathway. Life Sci. 2021;269:119090.
**ong R, Jiang W, Li N, Liu B, He R, Wang B, Geng Q. PM2.5-induced lung injury is attenuated in macrophage-specific NLRP3 deficient mice. Ecotoxicol Environ Saf. 2021;221:112433.
Li J, An Z, Song J, Du J, Zhang L, Jiang J, Ma Y, Wang C, Zhang J, Wu W. Fine particulate matter-induced lung in fl ammation is mediated by pyroptosis in mice. Ecotoxicol Environ Saf. 2021;219:112351.
Jia H, Liu Y, Guo D, He W, Zhao L, **a S. PM2.5-induced pulmonary inflammation via activating of the NLRP3/caspase-1 signaling pathway. Environ Toxicol. 2021;36:298–307.
Zhao J, Li M, Wang Z, Chen J, Zhao J, Xu Y, Wei X, Wang J, **e J. Role of PM2.5 in the development and progression of COPD and its mechanisms. Respir Res. 2019;20:120.
Wang Z, Zhao J, Wang T, Du X, **e J. Fine-particulate matter aggravates cigarette smoke extract-induced airway inflammation via Wnt5a-ERK pathway in COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:979–94.
Park S, Ra SW, Kang SY, Kim HC, Lee SW. Effect of particulate matter exposure on patients with COPD and risk reduction through behavioural interventions: the protocol of a prospective panel study. BMJ Open. 2020;10:e039394.
Kim H, Na G, Park S, Ra SW, Kang SY, Kim HC, Kim HC, Lee SW. The impact of life behavior and environment on particulate matter in chronic obstructive pulmonary disease. Environ Res. 2021;198:111265.
Huh J-Y, Kim H, Na G, Park S, Ra SW, Kang S-Y, Kim HC, Kim H-C, Lee SW. Effects of outdoors and indoors particulate matter 2.5 on COPD: Multicenter prospective observational study. Eur Respir J. 2021;58:PA1788.
Sun B, Shi Y, Li Y, Jiang J, Liang S, Duan J, Sun Z. Short-term PM(2.5) exposure induces sustained pulmonary fibrosis development during post-exposure period in rats. J Hazard Mater. 2020;385:121566.
Kim KH, Park TS, Kim YS, Lee JS, Oh YM, Lee SD, Lee SW. Resolvin D1 prevents smoking-induced emphysema and promotes lung tissue regeneration. Int J Chron Obstruct Pulmon Dis. 2016;11:1119–28.
Huh JW, Kim SY, Lee JH, Lee JS, Van Ta Q, Kim M, Oh YM, Lee YS, Lee SD. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol. 2011;301:L255–266.
Lee SH, Kim J, Kim NH, Kim OH, Shon CH, Kim SJ, Jang Y, Yun S, Lim SE, Jung SY, et al. Gut microbiota composition and metabolite profiling in smokers: a comparative study between emphysema and asymptomatic individuals with therapeutic implications. Thorax. 2023;78:1080–9.
Jang YO, Kim OH, Kim SJ, Lee SH, Yun S, Lim SE, Yoo HJ, Shin Y, Lee SW. High-fiber diets attenuate emphysema development via modulation of gut microbiota and metabolism. Sci Rep. 2021;11:7008.
Jang YO, Lee SH, Choi JJ, Kim DH, Choi JM, Kang MJ, Oh YM, Park YJ, Shin Y, Lee SW. Fecal microbial transplantation and a high fiber diet attenuates emphysema development by suppressing inflammation and apoptosis. Exp Mol Med. 2020;52:1128–39.
Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008;118:2771–84.
Charoud-Got J, Emma G, Seghers J, Tumba-Tshilumba MF, Santoro A, Held A, Snell J, Emteborg H. Preparation of a PM2.5-like reference material in sufficient quantities for accurate monitoring of anions and cations in fine atmospheric dust. Anal Bioanal Chem. 2017;409:7121–31.
Richter A, O’Donnell RA, Powell RM, Sanders MW, Holgate ST, Djukanovic R, Davies DE. Autocrine ligands for the epidermal growth factor receptor mediate interleukin-8 release from bronchial epithelial cells in response to cigarette smoke. Am J Respir Cell Mol Biol. 2002;27:85–90.
Knudsen L, Weibel ER, Gundersen HJ, Weinstein FV, Ochs M. Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. J Appl Physiol (1985). 2010;108:412–21.
Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;41:631–8.
Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012;8:1281–90.
Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z, Lin Y, Bai X, Liu X, Chen X, et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018;9:171.
Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:726–33.
Perotin JM, Adam D, Vella-Boucaud J, Delepine G, Sandu S, Jonvel AC, Prevost A, Berthiot G, Pison C, Lebargy F, et al. Delay of airway epithelial wound repair in COPD is associated with airflow obstruction severity. Respir Res. 2014;15:151.
Saetta M, Turato G, Baraldo S, Zanin A, Braccioni F, Mapp CE, Maestrelli P, Cavallesco G, Papi A, Fabbri LM. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med. 2000;161:1016–21.
Polosukhin VV, Richmond BW, Du RH, Cates JM, Wu P, Nian H, Massion PP, Ware LB, Lee JW, Kononov AV, et al. Secretory IgA Deficiency in Individual Small Airways is Associated with persistent inflammation and remodeling. Am J Respir Crit Care Med. 2017;195:1010–21.
Gohy ST, Hupin C, Fregimilicka C, Detry BR, Bouzin C, Gaide Chevronay H, Lecocq M, Weynand B, Ladjemi MZ, Pierreux CE, et al. Imprinting of the COPD airway epithelium for dedifferentiation and mesenchymal transition. Eur Respir J. 2015;45:1258–72.
Hei**k IH, Brandenburg SM, Postma DS, van Oosterhout AJ. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur Respir J. 2012;39:419–28.
Tatsuta M, Kan OK, Ishii Y, Yamamoto N, Ogawa T, Fukuyama S, Ogawa A, Fujita A, Nakanishi Y, Matsumoto K. Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: protective role of cathelicidin LL-37. Respir Res. 2019;20:251.
Aufderheide M, Scheffler S, Ito S, Ishikawa S, Emura M. Ciliatoxicity in human primary bronchiolar epithelial cells after repeated exposure at the air-liquid interface with native mainstream smoke of K3R4F cigarettes with and without charcoal filter. Exp Toxicol Pathol. 2015;67:407–11.
Yu Q, Chen X, Fang X, Chen Q, Hu C. Caveolin-1 aggravates cigarette smoke extract-induced MUC5AC secretion in human airway epithelial cells. Int J Mol Med. 2015;35:1435–42.
Andreoli C, Bassi A, Gregg EO, Nunziata A, Puntoni R, Corsini E. Effects of cigarette smoking on circulating leukocytes and plasma cytokines in monozygotic twins. Clin Chem Lab Med. 2015;53:57–64.
Scrimini S, Pons J, Agusti A, Soriano JB, Cosio BG, Torrecilla JA, Nunez B, Cordova R, Iglesias A, Jahn A, et al. Differential effects of smoking and COPD upon circulating myeloid derived suppressor cells. Respir Med. 2013;107:1895–903.
Wang J, Urbanowicz RA, Tighe PJ, Todd I, Corne JM, Fairclough LC. Differential activation of killer cells in the circulation and the lung: a study of current smoking status and chronic obstructive pulmonary disease (COPD). PLoS ONE. 2013;8:e58556.
De Falco G, Terlizzi M, Sirignano M, Commodo M, D’Anna A, Aquino RP, Pinto A, Sorrentino R. Human peripheral blood mononuclear cells (PBMCs) from smokers release higher levels of IL-1-like cytokines after exposure to combustion-generated ultrafine particles. Sci Rep. 2017;7:43016.
De Falco G, Colarusso C, Terlizzi M, Popolo A, Pecoraro M, Commodo M, Minutolo P, Sirignano M, D’Anna A, Aquino RP, et al. Chronic obstructive Pulmonary Disease-Derived circulating cells release IL-18 and IL-33 under Ultrafine Particulate Matter exposure in a Caspase-1/8-Independent manner. Front Immunol. 2017;8:1415.
Colarusso C, De Falco G, Terlizzi M, Roviezzo F, Cerqua I, Sirignano M, Cirino G, Aquino RP, Pinto A, D’Anna A, Sorrentino R. The inhibition of Caspase-1- does not revert particulate matter (PM)-Induced Lung Immunesuppression in mice. Front Immunol. 2019;10:1329.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5.
Liu J, Fan G, Tao N, Sun T. Role of Pyroptosis in respiratory diseases and its therapeutic potential. J Inflamm Res. 2022;15:2033–50.
Wang L, Chen Q, Yu Q, **ao J, Zhao H. TREM-1 aggravates chronic obstructive pulmonary disease development via activation NLRP3 inflammasome-mediated pyroptosis. Inflamm Res. 2021;70:971–80.
Su J, Ye Q, Zhang D, Zhou J, Tao R, Ding Z, Lu G, Liu J, Xu F. Joint association of cigarette smoking and PM2.5 with COPD among urban and rural adults in regional China. BMC Pulm Med. 2021;21:87.
Nakao M, Ishihara Y, Kim CH, Hyun IG. The impact of Air Pollution, including Asian sand dust, on respiratory symptoms and health-related quality of life in outpatients with chronic respiratory disease in Korea: a panel study. J Prev Med Public Health. 2018;51:130–9.
Cortez-Lugo M, Ramirez-Aguilar M, Perez-Padilla R, Sansores-Martinez R, Ramirez-Venegas A, Barraza-Villarreal A. Effect of personal exposure to PM2.5 on Respiratory Health in a Mexican panel of patients with COPD. Int J Environ Res Public Health. 2015;12:10635–47.
Camp PG, Ramirez-Venegas A, Sansores RH, Alva LF, McDougall JE, Sin DD, Pare PD, Muller NL, Silva CI, Rojas CE, Coxson HO. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43:725–34.
Chen X, Que C, Yao Y, Han Y, Zhang H, Li X, Lu X, Chen W, Hu X, Wu Y, et al. Susceptibility of individuals with lung dysfunction to systemic inflammation associated with ambient fine particle exposure: a panel study in Bei**g. Sci Total Environ. 2021;788:147760.
Garrod R, Marshall J, Barley E, Fredericks S, Hagan G. The relationship between inflammatory markers and disability in chronic obstructive pulmonary disease (COPD). Prim Care Respir J. 2007;16:236–40.
Agusti A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, Vestbo J, Lomas DA, Calverley PM, Wouters E, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS ONE. 2012;7:e37483.
Markelic I, Hlapcic I, Ceri A, Radic Antolic M, Samarzija M, Popovic-Grle S, Vukic Dugac A, Rumora L. Activation of NLRP3 inflammasome in stable chronic obstructive pulmonary disease. Sci Rep. 2022;12:7544.
Rehman AU, Shah S, Abbas G, Harun SN, Shakeel S, Hussain R, Hassali MAA, Rasool MF. Assessment of risk factors responsible for rapid deterioration of lung function over a period of one year in patients with chronic obstructive pulmonary disease. Sci Rep. 2021;11:13578.
Kim JK, Lee SH, Lee BH, Lee CY, Kim do J, Min KH, Kim SK, Yoo KH, Jung KS, Hwang YI. Factors associated with exacerbation in mild- to-moderate COPD patients. Int J Chron Obstruct Pulmon Dis. 2016;11:1327–33.
Mackay AJ, Kostikas K, Roche N, Frent SM, Olsson P, Pfister P, Gupta P, Patalano F, Banerji D, Wedzicha JA. Impact of baseline symptoms and health status on COPD exacerbations in the FLAME study. Respir Res. 2020;21:93.
Acknowledgements
We thank Sun-Hee Heo (Asan Medical Center), Shinhee Park (Gangneung Asan Hospital), Seung Won Ra (Ulsan University Hospital), and Sung-Yoon Kang (Gachon University Gil Medical Center) for their help with data collection.
Funding
This study was supported by grants from the research of the Korea Centers for Disease Control and Prevention [No. 2019ER671100 and 01, and 2021ER120900], the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (YJP & SJL), the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2023R1A2C2006688 and RS-2023-00222687, SWL), the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2022M3A9G8017220), Republic of Korea, and Medical Research Promotion Program through the Gangneung Asan Hospital funded by the Asan Foundation (2023II0003). The funder did not have any role in the design of the study and did not have any role in collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
CC, SYP, and SWL conceived and designed the study. SYP, J-YH, NHK, CHS, EYO, Y-JP, S-JL, and H-CK performed experiments and collected the data. CC, SYP, J-YH, and SWL analyzed data and drafted the manuscript. All authors revised and approved the final manuscript. All authors accept responsibility for the accuracy of the content of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal care and experimental procedures were approved by Institutional Animal Care and Use Committee of Asan Medical Center (approval number 2020-12-342). The previous clinical study was individually approved by the Institutional Review Boards as follows: Asan Medical Center (2019 − 0479), Gangneung Asan Hospital (2019-06-049), Ulsan University Hospital (2019-07-049), and Gachon University Gil Medical Center (GBirb2019-290). All participants were given detailed information about the study and provided written informed consent. The study is registered at ClinicalTrials.gov (Registration No. NCT04020237). The present study adhered to the Declaration of Helsinki and the ARRIVE guidelines, and all procedures were carried out in accordance with the relevant guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chung, C., Park, S.Y., Huh, JY. et al. Fine particulate matter aggravates smoking induced lung injury via NLRP3/caspase-1 pathway in COPD. J Inflamm 21, 13 (2024). https://doi.org/10.1186/s12950-024-00384-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12950-024-00384-z