Abstract
Background
Pneumocystis jirovecii (P. jirovecii) is an opportunistic fungus responsible for Pneumocystis pneumonia (PCP) in deeply immunocompromised patients and for pulmonary colonization in individuals with mild immunosuppression or impaired respiratory function. PCP and Cytomegalovirus (CMV) co-infections have been widely described whereas those involving other Herpesviruses (HVs) such as Epstein-Barr virus (EBV), Herpes simplex virus type 1 and type 2 (HSV-1 and -2), and Varicella zoster virus (VZV) remain scarce. To date, no data are available concerning HVs co-infections in P. jirovecii colonization.
Methods
Our main objective was to evaluate the frequency of HVs in bronchoalveolar lavage fluid (BALF) samples from patients with PCP or with pulmonary colonization. The secondary objective was to assess the relationship between HVs and the mortality rate in PCP patients. A retrospective single-center study over a seven-year period was conducted. All patients with P. jirovecii detected using PCR in a BALF sample and for whom a PCR assay for HVs detection was performed were included in the study.
Results
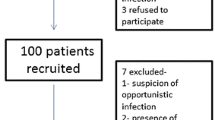
One hundred and twenty-five patients were included, corresponding to 77 patients with PCP and 48 colonized patients. At least one HV was detected in 54/77 (70.1%) PCP patients and in 28/48 (58.3%) colonized patients. EBV was the most frequent in both groups. Furthermore, the 30-day survival rate in PCP patients was significantly lower with [EBV + CMV] co-infection than that with EBV co-infection, [EBV + HSV-1] co-infection and without HV co-infection.
Conclusion
Our results show that the frequency of HV, alone or in combination is similar in PCP and colonization. They also suggest that [EBV + CMV] detection in BALF samples from PCP patients is associated with an increased mortality rate, underlying the significance to detect HVs in the course of PCP.
Similar content being viewed by others
Introduction
Pneumocystis jirovecii (P. jirovecii) is an opportunistic fungus responsible for Pneumocystis pneumonia (PCP) in deeply immunocompromised patients. Spontaneous evolution of PCP is fatal and this invasive fungal disease was a major cause of morbidity and mortality among HIV-infected people during the 80 and 90 s [1]. Nowadays, patients with hematological malignancy (HM), solid-organ tumor, solid-organ transplantation, primary immune deficiency and/or receiving long-term (> 3 months) or high-dose (> 0.5 mg/kg/day) corticosteroids or other immunosuppressive drugs present an increased risk of develo** PCP [2]. Thus, the at-risk population is now clearly recognized and PCP incidence in immunocompromised HIV-uninfected patients with no prophylaxis is estimated between 5 and 15% [3]. Pneumocystis detection in individuals with mild immunodeficiency or impaired respiratory function may also reflect pulmonary colonization. Pneumocystis colonization has been described in patients with chronic lung diseases such as chronic obstructive pulmonary disease but also occurs in immunosuppressed population [4]. Meanwhile, Herpesviridae/Herpesviruses (HVs), such as Cytomegalovirus (CMV), Epstein-Barr virus (EBV), Herpes simplex viruses type 1 and type 2 (HSV-1 and -2) or Varicella zoster virus (VZV) can also be responsible for severe pneumonia, especially in critically ill patients [5]. Moreover, rising numbers of critically ill patients are henceforth immunocompromised and account for approximately 30% of all ICU admissions [6]. Pulmonary co-infections with P. jirovecii and CMV have widely been described [7,8,9,10,11,12,12, 17]. Neutropenia induced by antiviral treatment (ganciclovir) would especially increase the risk of develo** co-infections [17].
There are conflicting reports regarding morbidity and mortality due to CMV co-infection during PCP. Some authors reported no significant difference of morbidity and mortality rates between PCP patients develo** a simultaneous CMV pneumonia and those with no viral pneumonia [9, 20,21,22,23]. However, the presence of CMV in BALF samples from HIV-infected patients with PCP has been correlated with a poor prognosis [10, 24] and numerous studies described a relationship between CMV infection and increased mortality rate in critically ill and SOT patients who developed PCP [11, 18, 25,26,27,28]. In the present study, we assessed the relationship between different HVs and the mortality rate. Although the 30-day mortality did not vary significantly according to the result of HV detection, our data indicated that the combination of [EBV + CMV] was significantly associated with an increased 30-day mortality rate in PCP patients compared to those without HV, with EBV, [EBV + CMV] and [EBV + HSV-1] (p = 0.0017). This increased mortality appears to be independent from the deep of the underlying immunodeficiency and rather linked to the specific [EBV + CMV] co-infection. Moreover, the absence of antiviral treatment was significantly associated with an increased day-30 mortality rate in PCP patients with at least one Herpesviridae detected in BALF. These results suggest that HV infections are important to test and treat in patients with PCP.
The diagnosis of pulmonary mixed infections remains challenging but is crucial because clinical manifestations are severe and associate with poor prognosis [14]. Pulmonary infections associating P. jirovecii and more than one viruses has already been described [13, 19, 27, 29]. Maartens et al. conducted a prospective study including 284 HIV-infected patients with pulmonary symptoms [30]. Simultaneous testing for community-acquired bacteria and viruses, mycobacteria, CMV and P. jirovecii was performed using PCR assays in induced sputa from all patients. Various respiratory viruses were detected in 203/284 patients (71.5%) with the highest prevalence in PCP patients (22/26, 85%). CMV was detected in 5/26 patients with PCP (19.2%) and multiple co-infections with respiratory viruses such as Human metapneumovirus A/B, Enterovirus, Influenza A or Parainfluenza virus were identified in PCP patients. In our study, Pneumocystis and HVs detections were performed in either a single BALF sample or two BALF samples collected less than two days apart. Thus, we demonstrated the simultaneous presence of HVs and P. jirovecii in 70.1% of PCP patients and in 58.3% of colonized patients. Moreover, at least two HVs were detected in one-third of BALF samples, regardless of the Pneumocystis status. In this context, syndromic molecular testing targeting the main pulmonary microorganisms represents an interesting method to reduce time and cost related to pre-analytical process. The usefulness of syndromic approach has already been assessed with a multiplex PCR TaqMan® array card (ThermoFisher) which simultaneously detects 24 viruses (including CMV and HSV 1 + 2), eight bacteria and two fungi (including P. jirovecii) [31] and with the “FTD respiratory pathogens 33” kit (Fast-Track Diagnostics, Esch-sur-Alzet, Luxembourg) targeting 33 microorganisms (including P. jirovecii and CMV) [30]. Recent studies proposed next-generation sequencing methods for a large-scale approach [13, 27, 29, 32]. These techniques allow thus prompt management of polymicrobial infections frequently associated with a poor prognosis.
Conclusion
Our study describes the detection of HVs in the lungs from Pneumocystis-infected patients with either PCP or colonization over a 7-year period. Distribution of each HV, alone or in combination was similar whatever the clinical form of Pneumocystis infection and EBV was the most frequent virus. Multiple viral infections were also observed in one-third of patient from each group. In addition, our results suggest that an increased 30-day mortality rate can be observed in PCP patients, especially in the case of [EBV + CMV] co-infection and in the absence of antiviral treatment.
Data availability
All data generated or analysed during this study are included in this published article.
References
Nevez G, Hauser PM, Le Gal S. Pneumocystis Jirovecii. Trends Microbiol. 2020;28:1034–5. https://doi.org/10.1016/j.tim.2020.03.006.
Sepkowitz KA. Opportunistic Infections in patients with and patients without acquired Immunodeficiency Syndrome. Clin Infect Dis. 2002;34:1098–107. https://doi.org/10.1086/339548.
Lagrou K, Chen S, Masur H, Viscoli C, Decker CF, Pagano L, et al. Pneumocystis Jirovecii Disease: basis for the revised EORTC/MSGERC invasive fungal Disease definitions in individuals without human immunodeficiency virus. Clin Infect Dis. 2021;72:114–20. https://doi.org/10.1093/cid/ciaa1805.
Morris A, Norris KA. Colonization by Pneumocystis Jirovecii and its role in Disease. Clin Microbiol Rev. 2012;25:297–317. https://doi.org/10.1128/CMR.00013-12.
Linssen CFM, Jacobs JA, Stelma FF, van Mook WNKA, Terporten P, Vink C, et al. Herpes simplex virus load in bronchoalveolar lavage fluid is related to poor outcome in critically ill patients. Intensive Care Med. 2008;34:2202–9. https://doi.org/10.1007/s00134-008-1231-4.
Azoulay E, Russell L, Van de Louw A, Metaxa V, Bauer P, Povoa P, et al. Diagnosis of severe Respiratory Infections in immunocompromised patients. Intensive Care Med. 2020;46:298–314. https://doi.org/10.1007/s00134-019-05906-5.
Vetter M, Battegay M, Trendelenburg M. Primary cytomegalovirus Infection with accompanying Pneumocystis Jirovecii Pneumonia in a patient with large-vessel vasculitis. Infection. 2010;38:331–4. https://doi.org/10.1007/s15010-010-0024-1.
Lee S, Park Y, Kim SG, Ko EJ, Chung BH, Yang CW. The impact of cytomegalovirus Infection on clinical severity and outcomes in kidney transplant recipients with pneumocystis jirovecii Pneumonia. Microbiol Immunol. 2020;64:356–65. https://doi.org/10.1111/1348-0421.12778.
Chou C-W, Lin F-C, Tsai H-C, Chang S-C. The impact of concomitant pulmonary Infection on immune dysregulation in Pneumocystis Jirovecii Pneumonia. BMC Pulm Med. 2014;14:182. https://doi.org/10.1186/1471-2466-14-182.
Yu Q, Jia P, Su L, Zhao H, Que C. Outcomes and prognostic factors of non-HIV patients with pneumocystis jirovecii Pneumonia and pulmonary CMV co-infection: a retrospective cohort study. BMC Infect Dis. 2017;17:392. https://doi.org/10.1186/s12879-017-2492-8.
Korkmaz Ekren P, Töreyin ZN, Nahid P, Doskaya M, Caner A, Turgay N, et al. The association between Cytomegalovirus co-infection with Pneumocystis Pneumonia and mortality in immunocompromised non-HIV patients. Clin Respir J. 2018;12:2590–7. https://doi.org/10.1111/crj.12961.
Hosseini-Moghaddam SM, Shokoohi M, Singh G, Dufresne SF, Boucher A, Jevnikar A, et al. A Multicenter Case-control study of the Effect of Acute rejection and cytomegalovirus Infection on Pneumocystis Pneumonia in Solid Organ Transplant recipients. Clin Infect Dis. 2019;68:1320–6. https://doi.org/10.1093/cid/ciy682.
**e Y, Ruan B, ** L, Zhu B. Case Report: next-generation sequencing in diagnosis of Pneumonia due to Pneumocystis Jirovecii and Cytomegalovirus in a patient with HIV Infection. Front Med (Lausanne). 2021;8:653294. https://doi.org/10.3389/fmed.2021.653294.
Fillatre P, Chevrier S, Revest M, Gacouin A, Jouneau S, Leroy H, et al. Human herpes virus co-infection is associated with mortality in HIV-negative patients with pneumocystis jirovecii Pneumonia. Eur J Clin Microbiol Infect Dis. 2013;32:189–94. https://doi.org/10.1007/s10096-012-1730-7.
Muramatsu H, Kuriyama A, Anzai Y, Ikegami T. A co-infection of varicella-zoster virus and pneumocystis jirovecii in a non-HIV immunocompromised patient: a case report. BMC Infect Dis. 2019;19:1092. https://doi.org/10.1186/s12879-019-4715-7.
Damiani C, Le Gal S, Da Costa C, Virmaux M, Nevez G, Totet A. Combined quantification of pulmonary pneumocystis jirovecii DNA and serum (1->3)-β-D-glucan for differential diagnosis of Pneumocystis Pneumonia and pneumocystis colonization. J Clin Microbiol. 2013;51:3380–8. https://doi.org/10.1128/JCM.01554-13.
Yong MK, Slavin MA, Kontoyiannis DP. Invasive fungal Disease and cytomegalovirus Infection: is there an association? Curr Opin Infect Dis. 2018;31:481–9. https://doi.org/10.1097/QCO.0000000000000502.
Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–22. https://doi.org/10.1001/jama.300.4.413.
Lécuyer R, Issa N, Tessoulin B, Lavergne R-A, Morio F, Gabriel F, et al. Epidemiology and clinical impact of respiratory coinfections at diagnosis of Pneumocystis Jirovecii Pneumonia. J Infect Dis. 2022;225:868–80. https://doi.org/10.1093/infdis/jiab460.
Miles PR, Baughman RP, Linnemann CC. Cytomegalovirus in the bronchoalveolar lavage fluid of patients with AIDS. Chest. 1990;97:1072–6. https://doi.org/10.1378/chest.97.5.1072.
Jacobson MA, Mills J, Rush J, Peiperl L, Seru V, Mohanty PK, et al. Morbidity and mortality of patients with AIDS and first-episode Pneumocystis carinii Pneumonia unaffected by concomitant pulmonary cytomegalovirus Infection. Am Rev Respir Dis. 1991;144:6–9. https://doi.org/10.1164/ajrccm/144.1.6.
Bozzette SA, Arcia J, Bartok AE, McGlynn LM, McCutchan JA, Richman DD, et al. Impact of Pneumocystis carinii and cytomegalovirus on the course and outcome of atypical Pneumonia in advanced human immunodeficiency virus Disease. J Infect Dis. 1992;165:93–8. https://doi.org/10.1093/infdis/165.1.93.
Kim T, Moon SM, Sung H, Kim M-N, Kim S-H, Choi S-H, et al. Outcomes of non-HIV-infected patients with Pneumocystis Pneumonia and concomitant pulmonary cytomegalovirus Infection. Scand J Infect Dis. 2012;44:670–7. https://doi.org/10.3109/00365548.2011.652665.
Benfield TL, Helweg-Larsen J, Bang D, Junge J, Lundgren JD. Prognostic markers of short-term mortality in AIDS-associated pneumocystis carinii Pneumonia. Chest. 2001;119:844–51. https://doi.org/10.1378/chest.119.3.844.
Zou J, Qiu T, Zhou J, Wang T, Ma X, ** Z, et al. Clinical manifestations and outcomes of renal transplantation patients with pneumocystis jirovecii Pneumonia and cytomegalovirus co-infection. Front Med (Lausanne). 2022;9:860644. https://doi.org/10.3389/fmed.2022.860644.
Frantzeskaki FG, Karampi E-S, Kottaridi C, Alepaki M, Routsi C, Tzanela M, et al. Cytomegalovirus reactivation in a general, nonimmunosuppressed intensive care unit population: incidence, risk factors, associations with organ dysfunction, and inflammatory biomarkers. J Crit Care. 2015;30:276–81. https://doi.org/10.1016/j.jcrc.2014.10.002.
Pan L, Wu F, Cai Q, Xu Z, Hu H, Tang T, et al. Whole genome profiling of Lung Microbiome in Solid Organ Transplant recipients reveals Virus involved Microecology May Worsen Prognosis. Front Cell Infect Microbiol. 2022;12:863399. https://doi.org/10.3389/fcimb.2022.863399.
Chiche L, Forel J-M, Roch A, Guervilly C, Pauly V, Allardet-Servent J, et al. Active cytomegalovirus Infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med. 2009;37:1850–7. https://doi.org/10.1097/CCM.0b013e31819ffea6.
Wang J, Han Y, Feng J. Metagenomic next-generation sequencing for mixed pulmonary Infection diagnosis. BMC Pulm Med. 2019;19:252. https://doi.org/10.1186/s12890-019-1022-4.
Maartens G, Griesel R, Dube F, Nicol M, Mendelson M. Etiology of Pulmonary Infections in Human Immunodeficiency Virus-infected inpatients using Sputum Multiplex Real-time polymerase chain reaction. Clin Infect Dis. 2020;70:1147–52. https://doi.org/10.1093/cid/ciz332.
Steensels D, Reynders M, Descheemaeker P, Curran MD, Hites M, Etienne I, et al. Epidemiology and clinical impact of viral, atypical, and fungal respiratory pathogens in symptomatic immunocompromised patients: a two-center study using a multi-parameter customized respiratory Taqman® array card. Eur J Clin Microbiol Infect Dis. 2019;38:1507–14. https://doi.org/10.1007/s10096-019-03579-y.
Lyu J, Deng Q, Li R, Tian B, Zhao Y, Hu X et al. Pneumonia caused by Coinfection with Cytomegalovirus and Pneumocystis Jirovecii in an HIV-Negative infant diagnosed by Metagenomic Next-Generation sequencing. IDR 2022;Volume 15:3417–25. https://doi.org/10.2147/IDR.S364241.
Acknowledgements
Thanks to Zuzana Saidak who provided technical help on statistical analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CD and AT designed the study. AR collected the data and designed the figures. AR and CD wrote the manuscript with support from AT and YLG. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was conducted using anonymous health care data in accordance with the French law and after approval from the local ethics committee (Direction de la Recherche Clinique et de l’Innovation, Amiens-Picardy University Hospital, Ref. PI2022_843_0011).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rucar, A., Totet, A., Le Govic, Y. et al. Pulmonary co-infections by Pneumocystis jirovecii and Herpesviridae: a seven-year retrospective study. Ann Clin Microbiol Antimicrob 23, 8 (2024). https://doi.org/10.1186/s12941-023-00663-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-023-00663-2