Abstract
Background
In bladder cancer, up to 70% of patients will relapse after resection within 5 years, in which the mechanism underlying the recurrence remains largely unclear.
Methods
Quantitative real-time PCR, western blot and immunohistochemistry were conducted. The assays of tumor sphere formation and tumor xenograft were further performed to assess the potential biological roles of ATF5 (activating transcription factor 5). Chromatin immunoprecipitation-qPCR and luciferase activity assays were carried out to explore the potential molecular mechanism. A two-tailed paired Student's t-test, χ2 test, Kaplan Meier and Cox regression analyses, and Spearman's rank correlation coefficients were used for statistical analyses.
Results
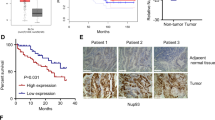
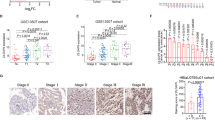
ATF5 is elevated in bladder urothelial cancer (BLCA) tissues, especially in recurrent BLCA, which confers a poor prognosis. Overexpressing ATF5 significantly enhanced, whereas silencing ATF5 inhibited, the capability of tumor sphere formation in bladder cancer cells. Mechanically, ATF5 could directly bind to and stimulate the promoter of DVL1 gene, resulting in activation of Wnt/β-catenin pathway.
Conclusions
This study provides a novel insight into a portion of the mechanism underlying high recurrence potential of BLCA, presenting ATF5 as a prognostic factor or potential therapeutic target for preventing recurrence in BLCA.
Similar content being viewed by others
Background
Globally, bladder cancer is the 10th most prevalent cancer type, with about 549,000 newly diagnosed patients in 2018 [1]. Unfortunately, up to 70% of these patients will relapse upon transurethral resection of bladder cancer [2], which greatly increases the suffering of patients.
Cancer recurrence is highly associated with cancer cell drug resistance and high tumorigenic capability [3]. Moreover, these characteristics could be examined in a small cell subpopulation in bladder cancer tissue, which are called the cancer stem cells (CSC) or the tumor initiating cells (TICs) [3,35, 36]. Abnormal activations of this pathway can lead to unrestrained cells proliferation and malignant transformation [35, 36]. As one of the most relevant pathways associated with TICs, this pathway is often abnormally stimulated in various cancers, including bladder cancer [13, 37]. Stimulation of Wnt/β-catenin pathway by miR-543-3p could increase [38], whereas inhibition of this signaling by miR-139-5p may inhibit [39] TIC-like phenotype of BLCA cells, supporting the vital roles of Wnt/β-catenin pathway in regulating TIC-like phenotype of bladder cancer. Consistent with these studies, we detected that Wnt/β-catenin signaling was abnormally activated in BLCA. We showed that ATF5 could directly target and positively regulate DVL1, leading to the stimulation of Wnt/β-catenin signaling.
DVL1, as a main component of the Wnt pathway, takes part in transduction of Wnt signals to β-catenin, and then stimulates downstream effector factors [40]. In this study, we found that ATF5 could directly bind to DVL1 promoter and stimulate its expression, and then activate the downstream genes of the Wnt/β-catenin pathway, including active β-catenin, MYC, CD44, JUN as well as CCND1, whereas down-regulating ATF5 reduced the expression of DVL1 and these factors. These findings demonstrate a novel mechanism underpinning hyperactivation of the Wnt/β-catenin pathway in BLCA. Herein, this study indicates that ATF5 could simulate the Wnt/β-catenin pathway and promote tumorigenic capability.
Conclusion
The present study reveals that overexpression of ATF5 in BLCA directly promotes DVL1 expression and stimulates the Wnt/β-catenin signaling, therefore increasing tumorigenicity, enhancing a TIC-like phenotype as well as predicting poor survival. Evaluation of the role of ATF5 in BLCA will broaden our understanding of the mechanism underpinning the high recurrence rate of BLCA, and establish whether ATF5 serves as a prognosis marker or potential treatment target for BLCA recurrence.
Availability of data and materials
The public datasets of BLCA in NCBI (https://www.ncbi.nlm.nih.gov/gene/); gene set enrichment analysis software program (GSEA, http://software.broadinstitute.org/gsea/msigdb/index.jsp).
References
de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180–90. https://doi.org/10.1016/S2214-109X(19)30488-7.
Chou R, Selph S, Buckley DI, Fu R, Griffin JC, Grusing S, Gore JL. Intravesical therapy for the treatment of nonmuscle invasive bladder cancer: a systematic review and meta-analysis. J Urol. 2017;197(5):1189–99. https://doi.org/10.1016/j.juro.2016.12.090.
Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–34. https://doi.org/10.1038/nm.4409.
Kurtova AV, **ao J, Mo Q, Pazhanisamy S, Krasnow R, Lerner SP, Chen F, Roh TT, Lay E, Ho PL, Chan KS. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517(7533):209–13. https://doi.org/10.1038/nature14034.
Wang S, Gao D, Chen Y. The potential of organoids in urological cancer research. Nat Rev Urol. 2017;14(7):401–14. https://doi.org/10.1038/nrurol.2017.65.
Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–68. https://doi.org/10.1038/nrc2499.
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–61. https://doi.org/10.1056/NEJMra061808.
Ohishi T, Koga F, Migita T. Bladder cancer stem-like cells: their origin and therapeutic perspectives. Int J Mol Sci. 2015. https://doi.org/10.3390/ijms17010043.
Li Y, Lin K, Yang Z, Han N, Quan X, Guo X, Li C. Bladder cancer stem cells: clonal origin and therapeutic perspectives. Oncotarget. 2017;8(39):66668–79. https://doi.org/10.18632/oncotarget.19112.
Li J, Yu B, Deng P, Cheng Y, Yu Y, Kevork K, Ramadoss S, Ding X, Li X, Wang CY. KDM3 epigenetically controls tumorigenic potentials of human colorectal cancer stem cells through Wnt/beta-catenin signalling. Nat Commun. 2017;8:15146. https://doi.org/10.1038/ncomms15146.
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111(2):241–50. https://doi.org/10.1016/s0092-8674(02)01014-0.
Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472(7341):110–4. https://doi.org/10.1038/nature09851.
Pierzynski JA, Hildebrandt MA, Kamat AM, Lin J, Ye Y, Dinney CP, Wu X. Genetic variants in the Wnt/beta-catenin signaling pathway as indicators of bladder cancer risk. J Urol. 2015;194(6):1771–6. https://doi.org/10.1016/j.juro.2015.07.032.
Li G, Xu Y, Guan D, Liu Z, Liu DX. HSP70 protein promotes survival of C6 and U87 glioma cells by inhibition of ATF5 degradation. J Biol Chem. 2011;286(23):20251–9. https://doi.org/10.1074/jbc.M110.211771.
Torres-Peraza JF, Engel T, Martin-Ibanez R, Sanz-Rodriguez A, Fernandez-Fernandez MR, Esgleas M, Canals JM, Henshall DC, Lucas JJ. Protective neuronal induction of ATF5 in endoplasmic reticulum stress induced by status epilepticus. Brain. 2013;136(Pt 4):1161–76. https://doi.org/10.1093/brain/awt044.
Greene LA, Lee HY, Angelastro JM. The transcription factor ATF5: role in neurodevelopment and neural tumors. J Neurochem. 2009;108(1):11–22. https://doi.org/10.1111/j.1471-4159.2008.05749.x.
Arias A, Lame MW, Santarelli L, Hen R, Greene LA, Angelastro JM. Regulated ATF5 loss-of-function in adult mice blocks formation and causes regression/eradication of gliomas. Oncogene. 2012;31(6):739–51. https://doi.org/10.1038/onc.2011.276.
Mason JL, Angelastro JM, Ignatova TN, Kukekov VG, Lin G, Greene LA, Goldman JE. ATF5 regulates the proliferation and differentiation of oligodendrocytes. Mol Cell Neurosci. 2005;29(3):372–80. https://doi.org/10.1016/j.mcn.2005.03.004.
Angelastro JM, Mason JL, Ignatova TN, Kukekov VG, Stengren GB, Goldman JE, Greene LA. Downregulation of activating transcription factor 5 is required for differentiation of neural progenitor cells into astrocytes. J Neurosci. 2005;25(15):3889–99. https://doi.org/10.1523/JNEUROSCI.3447-04.2005.
Angelastro JM, Ignatova TN, Kukekov VG, Steindler DA, Stengren GB, Mendelsohn C, Greene LA. Regulated expression of ATF5 is required for the progression of neural progenitor cells to neurons. J Neurosci. 2003;23(11):4590–600.
Nakamori D, Takayama K, Nagamoto Y, Mitani S, Sakurai F, Tachibana M, Mizuguchi H. Hepatic maturation of human iPS cell-derived hepatocyte-like cells by ATF5, c/EBPalpha, and PROX1 transduction. Biochem Biophys Res Commun. 2016;469(3):424–9. https://doi.org/10.1016/j.bbrc.2015.12.007.
Zhao Y, Zhang YD, Zhang YY, Qian SW, Zhang ZC, Li SF, Guo L, Liu Y, Wen B, Lei QY, Tang QQ, Li X. p300-dependent acetylation of activating transcription factor 5 enhances C/EBPbeta transactivation of C/EBPalpha during 3T3-L1 differentiation. Mol Cell Biol. 2014;34(3):315–24. https://doi.org/10.1128/MCB.00956-13.
Leong DT, Abraham MC, Gupta A, Lim TC, Chew FT, Hutmacher DW. ATF5, a possible regulator of osteogenic differentiation in human adipose-derived stem cells. J Cell Biochem. 2012;113(8):2744–53. https://doi.org/10.1002/jcb.24150.
Chen MK, Zhou JH, Wang P, Ye YL, Liu Y, Zhou JW, Chen ZJ, Yang JK, Liao DY, Liang ZJ, **e X, Zhou QZ, Xue KY, et al. BMI1 activates P-glycoprotein via transcription repression of miR-3682-3p and enhances chemoresistance of bladder cancer cell. Aging. 2021;13(14):18310–30. https://doi.org/10.18632/aging.203277.
Angelastro JM, Canoll PD, Kuo J, Weicker M, Costa A, Bruce JN, Greene LA. Selective destruction of glioblastoma cells by interference with the activity or expression of ATF5. Oncogene. 2006;25(6):907–16. https://doi.org/10.1038/sj.onc.1209116.
Rousseau J, Gagne V, Labuda M, Beaubois C, Sinnett D, Laverdiere C, Moghrabi A, Sallan SE, Silverman LB, Neuberg D, Kutok JL, Kra**ovic M. ATF5 polymorphisms influence ATF function and response to treatment in children with childhood acute lymphoblastic leukemia. Blood. 2011;118(22):5883–90. https://doi.org/10.1182/blood-2011-05-355560.
Monaco SE, Angelastro JM, Szabolcs M, Greene LA. The transcription factor ATF5 is widely expressed in carcinomas, and interference with its function selectively kills neoplastic, but not nontransformed, breast cell lines. Int J Cancer. 2007;120(9):1883–90. https://doi.org/10.1002/ijc.22469.
Ishihara S, Yasuda M, Ishizu A, Ishikawa M, Shirato H, Haga H. Activating transcription factor 5 enhances radioresistance and malignancy in cancer cells. Oncotarget. 2015;6(7):4602–14. https://doi.org/10.18632/oncotarget.2912.
Sheng Z, Ma L, Sun JE, Zhu LJ, Green MR. BCR-ABL suppresses autophagy through ATF5-mediated regulation of mTOR transcription. Blood. 2011;118(10):2840–8. https://doi.org/10.1182/blood-2010-12-322537.
Yu HB, Kunarso G, Hong FH, Stanton LW. Zfp206, Oct4, and Sox2 are integrated components of a transcriptional regulatory network in embryonic stem cells. J Biol Chem. 2009;284(45):31327–35. https://doi.org/10.1074/jbc.M109.016162.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. https://doi.org/10.1016/j.cell.2007.11.019.
Kumar SM, Liu S, Lu H, Zhang H, Zhang PJ, Gimotty PA, Guerra M, Guo W, Xu X. Acquired cancer stem cell phenotypes through Oct4-mediated dedifferentiation. Oncogene. 2012;31(47):4898–911. https://doi.org/10.1038/onc.2011.656.
Zhu F, Qian W, Zhang H, Liang Y, Wu M, Zhang Y, Zhang X, Gao Q, Li Y. SOX2 is a marker for stem-like tumor cells in bladder cancer. Stem Cell Rep. 2017;9(2):429–37. https://doi.org/10.1016/j.stemcr.2017.07.004.
Sedaghat S, Gheytanchi E, Asgari M, Roudi R, Keymoosi H, Madjd Z. Expression of cancer stem cell markers OCT4 and CD133 in transitional cell carcinomas. Appl Immunohistochem Mol Morphol. 2017;25(3):196–202. https://doi.org/10.1097/PAI.0000000000000291.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. https://doi.org/10.1146/annurev.cellbio.20.010403.113126.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205. https://doi.org/10.1016/j.cell.2012.05.012.
Mohammed MK, Shao C, Wang J, Wei Q, Wang X, Collier Z, Tang S, Liu H, Zhang F, Huang J, Guo D, Lu M, Liu F, et al. Wnt/beta-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016;3(1):11–40. https://doi.org/10.1016/j.gendis.2015.12.004.
Gao RL, Chen XR, Li YN, Yan XY, Sun JG, He QL, Cai FZ. Upregulation of miR-543-3p promotes growth and stem cell-like phenotype in bladder cancer by activating the Wnt/beta-catenin signaling pathway. Int J Clin Exp Pathol. 2017;10(9):9418–26.
Luo H, Yang R, Li C, Tong Y, Fan L, Liu X, Xu C. MicroRNA-139-5p inhibits bladder cancer proliferation and self-renewal by targeting the Bmi1 oncogene. Tumour Biol. 2017;39(7):1393371250. https://doi.org/10.1177/1010428317718414.
Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22(5):717–27. https://doi.org/10.1016/j.cellsig.2009.11.021.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China (No. 81902991), Guangzhou Science and Technology Planning Project (No. 202102021056) and Youth Project of The Third Affiliated Hospital of Southern Medical University (No. QD2019N010).
Author information
Authors and Affiliations
Contributions
LCD and ZT designed the study. ZJH, TH and ZX carried out the experiments of cytobiology. XZY, CTY and YHY conducted the follow-up study. CQ, YJK and ZQZ participated in acquisition of data. XKY, WHY and CMK took part in collection of clinical samples. GWB, XM and BJM analyzed the experimental data. ZJH drafted the manuscript. YC, DHF and HZP participated in the revising of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study involved in human specimens was approved by the Clinical Trials Ethics Committee (The Third Affiliated Hospital of Southern Medical University, and Affiliated Cancer Hospital & Institute of Guangzhou Medical University). This study involved in animals was approved by the Ethics Committee of Experimental Animals, Southern Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, J., Tian, H., Zhi, X. et al. Activating transcription factor 5 (ATF5) promotes tumorigenic capability and activates the Wnt/b-catenin pathway in bladder cancer. Cancer Cell Int 21, 660 (2021). https://doi.org/10.1186/s12935-021-02315-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-02315-x