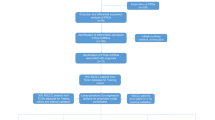
Abstract
Background
Long non-coding RNAs (lncRNAs) are increasingly recognized as the crucial mediators in the regulation of ferroptosis and iron metabolism. A systematic understanding of ferroptosis and iron-metabolism related lncRNAs (FIRLs) in lung adenocarcinoma (LUAD) is essential for new diagnostic and therapeutic strategies.
Methods
FIRLs were obtained through Pearson correlation analysis between ferroptosis and iron-metabolism related genes and all lncRNAs. Univariate and multivariate Cox regression analysis were used to identify optimal prognostic lncRNAs. Next, a novel signature was constructed and risk score of each patient was calculated. Survival analysis and ROC analysis were performed to evaluate the predictive performance using The Cancer Genome Atlas Lung Adenocarcinoma (TCGA-LUAD) and Gene Expression Omnibus (GEO) datasets, respectively. Furthermore, multivariate Cox and stratification analysis were used to assess prognostic value of this signature in whole cohort and various subgroups. The correlation of risk signature with immune infiltration and gene mutation was also discussed. The expression of lncRNAs was verified by quantitative real-time PCR (qRT-PCR).
Results
A 7-FIRLs signature including ARHGEF26-AS1, LINC01137, C20orf197, MGC32805, TMPO-AS1, LINC00324, and LINC01116 was established in the present study to assess the overall survival (OS) of LUAD. The survival analysis and ROC curve indicated good predictive performance of the signature in both the TCGA training set and the GEO validation set. Multivariate Cox and stratification analysis indicated that the 7‐FIRLs signature was an independent prognostic factor for OS. Nomogram exhibited robust validity in prognostic prediction. Differences in immune cells, immune functions and gene mutation were also found between high-risk and low-risk groups.
Conclusions
This risk signature based on the FIRLs may be promising for the clinical prediction of prognosis and immunotherapeutic responses in LUAD patients.
Similar content being viewed by others
Background
Lung cancer, an extremely heterogeneous disease, caused more deaths in 2017 than breast, prostate, colorectal, and brain cancers combined [29]. LUAD is one of the important sub-types of lung cancer with an increasing incidence [28]. Despite great efforts having been made in develo** novel treatments but still received a poor prognosis with 5-year survival rates vary from 4% to 17% [13]. Patients with histologically similar tumors may have different outcomes due to molecular differences. Therefore, there is an urgent need to find new sensitive biomarkers for predicting survival of LUAD patients. Compared with a single biomarker, integrating multiple biomarkers into a signature would greatly improve prognostic prediction.
Iron is an essential trace element for human body. Its deficiency or excess can influence many biological processes [23]. Cancer cells exhibit an enhanced dependence on iron for growth and are dramatically more susceptible to iron depletion than non-cancer cells [21]. However, highly increased iron concentrations result in cell death through membrane lipid peroxidation, termed ferroptosis [12, 31]. Ferroptosis is an iron-dependent pathway of cell death that was discovered in recent years [18, 19]. The induction of cell death is known to be an viable approach for cancer therapy. Ferroptosis has also been identified as a potential prevention or therapeutic strategies to trigger cancer cell death, especially for malignancies that are resistant to traditional treatments [20]. Some studies have noticed the potential function of ferroptosis and iron metabolism in lung cancer development and suppression, but the detailed regulators remain unclear. Meanwhile, lncRNAs are defined as non-protein-coding transcripts larger than 200 nucleotides to distinguish them from small noncoding RNAs [16]. LncRNAs are participated in various biological purposes, such as immune, metabolism, infection, and so on. LncRNAs have been shown to function as master regulators in various disease processes including cancer [11]. Remarkably, it has been found that lncRNAs are the crucial mediators in the regulation of ferroptosis and iron metabolism in cancer [17]. In routine clinical practice, pathologic staging is a vital prognostic determinant of LUAD. However, clinical outcomes differ among patients at the same stage, indicating that the traditional staging system cannot adequately predict the prognosis of patients. Biomarkers related to tumor diagnosis and prognosis urgently need to be developed. Disturbances in iron metabolism cause excessive intracellular iron storage and may induce ferroptosis [4]. Impaired ferroptosis is implicated in various pathological conditions [7]. Due to the important role of ferroptosis and iron metabolism in cancer, its related lncRNA has also attracted a lot of attention [24].
To the best of our knowledge, this study is the first one to identify and comprehensively analyze prognostic FIRLs in LUAD. This signature based on 7 FIRLs provides a useful tool to supplement the traditional clinical prognostic factors, and guides prognostic prediction and therapeutic decisions. Additionally, we provide a FIRLs-related nomogram combining clinical factors to predict the OS of LUAD patients with an effective quantitative approach.
Immune regulation plays a crucial part in the progression of LUAD. The number and proportion of infiltrating immune cells are recognized as important factors affecting cancer progression and immunotherapy response and associated with patient prognosis. According to the tumor immunoediting hypothesis [9], less immunogenic cancer cells are selected for during tumor development in immune-competent hosts, to evade antitumor immune responses. This may result in increased immunosuppressive cells (e.g., regulatory T cells), decreased immunoreactive cells (e.g., helper T cells). Thus, we hypothesized that patients in different risk groups would have different immunotherapeutic responses. Results found that high-risk LUAD patients had higher NK cells infiltration and lower fractions of Mast cells and helper T cells than low-risk patients. The above results suggest that the poorer prognosis of high-risk patients is due to higher immunosuppression and lower immunoreactivity in the tumor microenvironment, and these differences contribute to tumor progression. Checkpoint inhibitor-based immunotherapies have improved the survival of patients of advanced malignancies [14]. Significant differences in the expression of immune checkpoints between high and low risk groups suggested the differences in the sensitivity to immunotherapies. Furthermore, findings in some cancer suggest that TMB may predict clinical response to immune checkpoint inhibitors [26]. In this study, we found that patients with LUAD in high risk group had higher TMB levels which was related to the immune effect.
However, several limitations of our study should be taken into consideration. Firstly, our study was mainly based on data from TCGA in which most patients were White or Asian. Caution must be taken when extrapolating our findings to patients from other ethnicities. Secondly, external validation of the signature in large-scale multicenter cohorts is necessary. Thirdly, further functional experiments in our laboratory will be required to verify findings and elucidate the roles of FIRLs in LUAD. In addition to its excellent performance in differentiating LUAD from normal lung, the role of the signature in differentiating normal lung, pulmonary nodules, and small cell lung cancer remains to be further elucidated.
In summary, the 7-FIRLs signature is a potential tool for predicting the OS rate of LUAD patients. Importantly, the signature might be associated with immune infiltration levels and even the TMB scores. We expect this robust signature will provide clues on biological behaviors as well as prognostic characteristics in clinical tests.
Conclusions
By and large, we successfully constructed a strong predictive signature of ferroptosis and iron metabolism which may serve as a new biomarker and therapeutic target affecting the progression of LUAD. Meanwhile, the signature helps researchers deeply understand the correlation between ferroptosis and tumourigenesis. Furthermore, this study provides a promising avenue for future anti-tumor immunotherapy.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional files.
Abbreviations
- LNCRNAs:
-
Long non-coding RNAs
- FIRLs:
-
Ferroptosis and iron-metabolism related LncRNAs
- LUAD:
-
Lung adenocarcinoma
- TCGA-LUAD:
-
The Cancer Genome Atlas Lung Adenocarcinoma
- GEO:
-
Gene Expression Omnibus
- qRT-PCR:
-
Quantitative real-time PCR
- OS:
-
Overall survival
- TMB:
-
Tumor mutation burden
- MAF:
-
Mutation annotation format
- MSigDB:
-
Molecular Signatures Database
- AUC:
-
Area under the curve
- PCA:
-
Principal component analysis
- HR:
-
Hazard ratios
- CI:
-
Confidence intervals
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- STR:
-
Short tandem repeat
- FBS:
-
Fetal bovine serum
- SNP:
-
Single nucleotide polymorphism
- INS:
-
Insertion
- DEL:
-
Deletion
- NGS:
-
Next Generation Sequencing
References
Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18(1):220. https://doi.org/10.1186/s13059-017-1349-1.
Abdelaal AM, Attalla EM, Elshemey MW. Estimation of out-of-field dose variation using markus ionization chamber detector. Sci Med J. 2020;2(1):8–15. https://doi.org/10.28991/SciMedJ-2020-0201-2.
Agsalda-Garcia M, Shieh T, Souza R, Kamada N, Loi N, Oda R, Acosta-Maeda T, Choi SY, Lim E, Misra A, Shiramizu B. Raman-enhanced spectroscopy (RESpect) probe for childhood non-Hodgkin lymphoma. Sci Med J. 2020;2(1):1–7. https://doi.org/10.28991/SciMedJ-2020-0201-1.
Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci. 2016;41(3):274–86. https://doi.org/10.1016/j.tibs.2015.11.012.
Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman C, Fridman WH, de Reynies A. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17(1):218. https://doi.org/10.1186/s13059-016-1070-5.
Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–59. https://doi.org/10.1007/978-1-4939-7493-1_12.
Chen X, Yu C, Kang R, Tang D. Iron metabolism in ferroptosis. Front Cell Dev Biol. 2020;8: 590226. https://doi.org/10.3389/fcell.2020.590226.
Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–89. https://doi.org/10.1101/gr.132159.111.
Efremova M, Rieder D, Klepsch V, Charoentong P, Finotello F, Hackl H, Hermann-Kleiter N, Löwer M, Baier G, Krogsdam A, Trajanoski Z. Targeting immune checkpoints potentiates immunoediting and changes the dynamics of tumor evolution. Nat Commun. 2018;9(1):32. https://doi.org/10.1038/s41467-017-02424-0.
Finotello F, Mayer C, Plattner C, Laschober G, Rieder D, Hackl H, et al. Molecular and pharmacological modulators of the tumor immune contexture revealed by deconvolution of RNA-seq data. Genome Med. 2019;11(1):34. https://doi.org/10.1186/s13073-019-0638-6.
Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. https://doi.org/10.1186/1476-4598-10-38.
Hassannia B, Vandenabeele P, VandenBerghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830–49. https://doi.org/10.1016/j.ccell.2019.04.002.
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. https://doi.org/10.1016/S0140-6736(16)30958-8.
Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33(5):843–52. https://doi.org/10.1016/j.ccell.2018.03.018.
** D, Song Y, Chen Y, Zhang P. Identification of a seven-lncRNA immune risk signature and construction of a predictive nomogram for lung adenocarcinoma. Biomed Res Int. 2020;2020:7929132. https://doi.org/10.1155/2020/7929132.
Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407. https://doi.org/10.1016/j.cell.2018.01.011.
Kosvyra A, Maramis C, Chouvarda I. Develo** an integrated genomic profile for cancer patients with the use of NGS data. ESJ. 2019;3(3):157–67. https://doi.org/10.28991/esj-2019-01178.
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. https://doi.org/10.1038/s41419-020-2298-2.
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–14. https://doi.org/10.1093/nar/gkaa407.
Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater. 2019;31(51): e1904197. https://doi.org/10.1002/adma.201904197.
Manz DH, Blanchette NL, Paul BT, Torti FM, Torti SV. Iron and cancer: recent insights. Ann N Y Acad Sci. 2016;1368(1):149–61. https://doi.org/10.1111/nyas.13008.
Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28(11):1747–56. https://doi.org/10.1101/gr.239244.118.
Musallam KM, Taher AT. Iron deficiency beyond erythropoiesis: should we be concerned? Curr Med Res Opin. 2018;34(1):81–93. https://doi.org/10.1080/03007995.2017.1394833.
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34. https://doi.org/10.1186/s13045-019-0720-y.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–7. https://doi.org/10.1038/nmeth.3337.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. https://doi.org/10.1126/science.aaa1348.
Racle J, Gfeller D. EPIC: a tool to estimate the proportions of different cell types from bulk gene expression data. Methods Mol Biol. 2020;2120:233–48. https://doi.org/10.1007/978-1-0716-0327-7_17.
Shiraishi K, Kunitoh H, Daigo Y, Takahashi A, Goto K, Sakamoto H, et al. A genome-wide association study identifies two new susceptibility loci for lung adenocarcinoma in the Japanese population. Nat Genet. 2012;44(8):900–3. https://doi.org/10.1038/ng.2353.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590.
Wang M, Mao C, Ouyang L, Liu Y, Lai W, Liu N, et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26(11):2329–43. https://doi.org/10.1038/s41418-019-0304-y.
Wang S, Luo J, Zhang Z, Dong D, Shen Y, Fang Y, et al. Iron and magnetic: new research direction of the ferroptosis-based cancer therapy. Am J Cancer Res. 2018;8(10):1933–46.
Wang Z, Chen X, Liu N, Shi Y, Liu Y, Ouyang L, Tam S, **ao D, Liu S, Wen F, Tao Y. A nuclear long non-coding RNA LINC00618 accelerates ferroptosis in a manner dependent upon apoptosis. Mol Ther. 2021;29(1):263–74. https://doi.org/10.1016/j.ymthe.2020.09.024.
Wu Y, Zhang S, Gong X, Tam S, **ao D, Liu S, Tao Y. The epigenetic regulators and metabolic changes in ferroptosis-associated cancer progression. Mol Cancer. 2020;19(1):39. https://doi.org/10.1186/s12943-020-01157-x.
Zhang X, Han J, Du L, Li X, Hao J, Wang L, et al. Unique metastasis-associated lncRNA signature optimizes prediction of tumor relapse in lung adenocarcinoma. Thorac Cancer. 2020;11(3):728–37. https://doi.org/10.1111/1759-7714.13325.
Zhou M, Shao W, Dai H, Zhu X. A robust signature based on autophagy-associated LncRNAs for predicting prognosis in lung adenocarcinoma. Biomed Res Int. 2020;2020:3858373. https://doi.org/10.1155/2020/3858373.
Zhou N, Bao J. FerrDb: a manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database. 2020. https://doi.org/10.1093/database/baaa021.
Acknowledgements
We are sincerely acknowledge the contributions from the TCGA project and the GEO project.
Funding
This work was supported by grants from the Major Scientific and Technological Innovation Project of Shandong Province (2018CXGC1212), the CSCO-Qilu Cancer Research Fund (Y-Q201802-014), the Medical and Health Technology Innovation Plan of **an City (201805002).
Author information
Authors and Affiliations
Contributions
JY conceived the study and drafted the manuscript; XC, XL and XZ collected the data; RL confirmed and sorted out the data; YQ revised this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Identification of FIRLs. A, B The correlation networks of 296 ferroptosis and iron metabolism related genes (red) and lncRNAs (green) from TCGA (A) and GSE37745 (B). C Venn diagram showed the intersection FIRLs from TCGA and GSE37745. FIRLs, ferroptosis and iron-metabolism related lncRNAs.
Additional file 2: Figure S2.
Functional enrichment analysis and ferroptosis correlation analysis. A A Sankey diagram was depicted to visualize the correlation of lncRNAs, mRNAs, and risk type. B, C Results for GO (B) and KEGG (C) enrichment analysis of the mRNAs related with the 7 FIRLs. “BP”: biological process, “CC”: cellular component, and “MF”: molecular function. E–L The correlation expression between 7 FIRLs and four most common ferroptosis-related mRNAs (FTH1, GPX4, ACSL4, PTGS2). FIRLs ferroptosis and iron metabolism related lncRNAs, GO gene ontology, KEGG Kyoto Encyclopedia of Genes and Genomes.
Additional file 3: Figure S3.
Scatter plots showed the relationship between the prognostic signature and immune cell infiltration. A–F The relationship between risk score and immune cell infiltration. G–N The relationship between expression level of a single lncRNA in the signature and immune cell infiltration.
Additional file 4: Figure S4.
Somatic mutation information of LUAD patients. A Waterfall plots represent mutation information of each gene in LUAD patients. The small rectangles with different color represent different mutation types. B–D Classification of different mutation types, in which missense mutation was the most common type, SNP occurred more proportion than INS or DEL, and C > A was the most common of SNV. E The number of variants per sample. F The box diagram showed the mutation type with different colors. G The top ten mutated genes in LUAD. SNP single nucleotide polymorphism, INS insertion, DEL deletion.
Additional file 5: Table S1.
Ferroptosis and iron-metabolism related genes.
Additional file 6: Table S2.
Primers sequences in qRT-PCR.
Additional file 7: Table S3.
118 Ferroptosis and iron-metabolism related lncRNAs.
Additional file 8: Table S4.
The results of univariate and multivariate Cox regression analysis performed to compare prognostic value of previously published signatures with LncSig developed in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yao, J., Chen, X., Liu, X. et al. Characterization of a ferroptosis and iron-metabolism related lncRNA signature in lung adenocarcinoma. Cancer Cell Int 21, 340 (2021). https://doi.org/10.1186/s12935-021-02027-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-02027-2