Abstract
Background
(+)-Nootkatone is a highly valued sesquiterpenoid compound, exhibiting a typical grapefruit aroma and various desired biological activities for use as aromatics and pharmaceuticals. The high commercial demand of (+)-nootkatone is predominately met by chemical synthesis, which entails the use of environmentally harmful reagents. Efficient synthesis of (+)-nootkatone via biotechnological approaches is thus urgently needed to satisfy its industrial demand. However, there are only a limited number of studies that report the de novo synthesis of (+)-nootkatone from simple carbon sources in microbial cell factories, and with relatively low yield.
Results
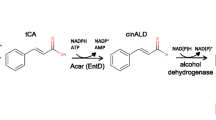
As the direct precursor of (+)-nootkatone biosynthesis, (+)-valencene was first produced in large quantities in Saccharomyces cerevisiae by overexpressing (+)-valencene synthase CnVS of Callitropsis nootkatensis in combination with various mevalonate pathway (MVA) engineering strategies, including the expression of CnVS and farnesyl diphosphate synthase (ERG20) as a fused protein, overexpression of a truncated form of the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase (tHMG1), and downregulating the squalene synthase enzyme (ERG9). These approaches altogether brought the production of (+)-valencene to 217.95 mg/L. Secondly, we addressed the (+)-valencene oxidation by overexpressing the Hyoscyamus muticus premnaspirodiene oxygenase (HPO) variant (V482I/A484I) and cytochrome P450 reductase (ATR1) from Arabidopsis thaliana. However, (+)-valencene was predominantly oxidized to β-nootkatol and only minor amounts of (+)-nootkatone (9.66 mg/L) were produced. We further tackled the oxidation of β-nootkatol to (+)-nootkatone by screening various dehydrogenases. Our results showed that the short-chain dehydrogenase/reductase (SDR) superfamily dehydrogenases ZSD1 of Zingiber zerumbet and ABA2 of Citrus sinensis were capable of effectively catalyzing β-nootkatol oxidation to (+)-nootkatone. The yield of (+)-nootkatone increased to 59.78 mg/L and 53.48 mg/L by additional overexpression of ZSD1 and ABA2, respectively.
Conclusion
We successfully constructed the (+)-nootaktone biosynthesis pathway in S. cerevisiae by overexpressing the (+)-valencene synthase CnVS, cytochrome P450 monooxygenase HPO, and SDR family dehydrogenases combined with the MVA pathway engineering, providing a solid basis for the whole-cell production of (+)-nootkatone. The two effective SDR family dehydrogenases tested in this study will serve as valuable enzymatic tools in further optimizing (+)-nootkatone production.
Similar content being viewed by others
Background
Sesquiterpenes are a large group of terpenoids and generally found in the plant essential oils [1, 2]. Many of them show a unique odor with a very low perception threshold, which draws intense interest from the beverage, cosmetic, and medical industries [1, 2]. The oxidized sesquiterpene (+)-nootkatone exhibits a typical grapefruit aroma at a very low threshold of about 1 μg/L [3, 4]. Notably, (+)-nootkatone has also been reported to show interesting therapeutic potentials, such as anticancer, antiplatelet aggregation, antimicrobial, and anti-inflammation activities, and thus represents a promising drug precursor [3, 4]. (+)-Nootkatone was initially isolated from the heartwood of Alaska Yellow Cedar and was later also found to be constituent of essential oils from grapefruits and pummelo [4]. However, (+)-nootkatone is only present in trace amounts in these plants and natural extraction is proven to be very inefficient, limiting its commercial applications. Although chemical oxidation of (+)-valencene has been employed to produce (+)-nootkatone to satisfy the high industry demand, they involve the use of environment-unfriendly oxidizing reagents such as tert-butyl peracetate, tert-butyl hydroperoxide, or heavy metals [5, 6]. With the development of synthetic biology, constructing microbial cell factories represents a promising alternative for the production of (+)-nootkatone [1].
The common precursor for terpenoid synthesis is isopentenyl diphosphate (IPP), which is derived from the mevalonate pathway (MVA) or the methyl-d-erythritol phosphate (MEP) pathway [7,8,9]. Condensation of IPP and its isomers dimethylallyl pyrophosphate (DMAPP) results in geranyl pyrophosphate (GPP) and farnesyl diphosphate (FPP), which are the precursors for monoterpene and sesquiterpene synthesis, respectively [1, 8]. Saccharomyces cerevisiae naturally synthesizes FPP through the MVA pathway and has thus been used for enhanced production of industrially relevant terpenoids by introducing the corresponding heterologous terpene synthase in combination with several metabolic engineering approaches [1]. The synthesized terpene can be further modified by regio- and stereo-specific oxidation, reduction, and acetylation et al., generating structurally diverse terpenoid compounds [1, 10].
As the direct precursor of (+)-nootkatone, ample supply of (+)-valencene is a prerequisite for efficient (+)-nootkatone synthesis [4, 10]. Up to now, only several (+)-valencene synthases have been identified and tested for (+)-valencene biosynthesis, including VvVal of Vitis vinifera [11], Cstps1 of Citrus sinensis [12], GFTpsD of Citrus × paradis [13], and CnVS of C. nootkatensis [13]. Among others, CnVS is proven to be the most robust one regarding catalytic pH and temperature, which is a desired property for the application in different hosts or under various physiological conditions [13]. Overexpression of CnVS in yeast strain WAT11, however, gave rise to only 1.36 mg/L of (+)-valencene [13]. (+)-Valencene production up to 3 mg/L has been achieved by expressing the (+)-valencene synthase GFTpsD and simultaneously downregulating the squalene synthase in S. cerevisiae [14]. Apart from yeast, (+)-valencene production has also been attempted in Corynebacterium glutamicum [15] and Schizophyllum commune [16], yielding 2.41 mg/L and 16 mg/L valencene, respectively. Thus, the yield of (+)-valencene in different hosts is still too low to reach the industrial demand, hindering its further oxidation for (+)-nootkatone biosynthesis.
The biosynthesis of (+)-nootkatone has been described by either oxidizing the exogenously added (+)-valencene by whole-cell catalysts or simultaneous expression of the (+)-valencene oxidases in (+)-valencene production strains [4]. Several cytochrome P450 enzymes, including CYP109B1 of Bacillus subtilis [17], CYP71D51v2 of tobacco [10], CYP71D4 of Solanum tuberosum [10], CYP71AV8 of Cichorium intybus [18], and the premnaspirodiene oxygenase of Hyoscyamus muticus (CYP71D55, HPO) [19] have been overexpressed in yeast and employed as whole-cell catalysts to catalyze the oxidation of (+)-valencene. Further enzyme engineering of HPO targeting its substrate recognition site has identified an HPO variant (HPO V482I/A484I) with a fivefold improved catalytic efficiency in the oxidation of (+)-valencene. However, these P450 enzymes mostly generate β-nootkatol as the predominant product and produce only minor amounts of (+)-nootkatone [18, 20]. On the other hand, a lipoxygenase ValOx from Pleurotus sapidus was found to catalyze the oxidation of (+)-valencene primarily to (+)-nootkatone [21, 22], although it has not been explored further for its applicability in (+)-nootkatone biosynthesis in a microbial cell factory. More recently, an alcohol dehydrogenase from Pichia pastoris has been found to be capable of converting β-nootkatol to (+)-nootkatone [23]. Nevertheless, efficient and selective oxidation of (+)-valencene to nootakatone is still challenging, which represents another bottleneck in (+)-nootkatone biosynthesis to meet the high industrial demand.
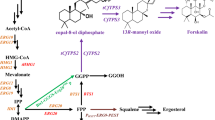
Although (+)-nootkatone production in microbial cell factories has been attempted, the yields are unacceptably low and development of an economically viable bioprocess for (+)-nootkatone production still remains challenging. In this study, we constructed the nootaktone biosynthesis pathway in S. cerevisiae by overexpressing the (+)-valencene synthase CnVS and the P450 enzyme HPO in combination with engineering the endogenous MVA pathway (Fig. 1). Importantly, several dehydrogenases were screened for catalyzing the conversion of β-nootkatol to (+)-nootkatone. The yield of (+)-nootkatone reached 59.78 mg/L in our constructed S. cerevisiae cell factory, thus providing a solid basis for high-level production of (+)-nootkatone.
Scheme of (+)-nootkatone biosynthesis based on the MVA pathway in S. cerevisiae. To increase the metabolic flux of the MVA pathway in S. cerevisiae, tHMG1 was overexpressed and the competitive ERG9 pathway was dynamically controlled by replacing its endogenous promoter with PHXT1. ERG20 was simultaneously fused with CnVS of C. nootkatensis and overexpressed to channel the metabolic flux to (+)-valencene production. The production of (+)-nootkatone was achieved by the overexpression of HPO, ATR1, and the indicated dehydrogenases
Results
Production of (+)-valencene by CnVS expression in S. cerevisiae
Saccharomyces cerevisiae has been proven to be an ideal host for the production of highly-valued terpenoid compounds by leveraging its inherent capacity to synthesize terpenoid precursors through the MVA pathway [1, 2]. As (+)-valencene is the precursor for (+)-nootkatone biosynthesis, we first explored the biosynthesis of (+)-valencene in S. cerevisiae W303 by expressing the robust (+)-valencene synthase CnVS (strain V02). n-Dodecane (10%, v/v) was added to the fermentation medium for in situ separation of the produced (+)-valencene given the low solubility of (+)-valencene in the aqueous phase and its high volatility [15]. Our growth test revealed that 10% n-dodecane showed only a marginal effect while (+)-valencene up to 500 mg/ml elicited hardly any inhibitory effect on the yeast growth (Additional file 1: Fig. S1). GC–MS analysis of the n-dodecane phase procured from the V02 cultivation revealed (+)-valencene as the dominant product, reaching about 11.6 mg/L (Fig. 2). The mass spectrum of the produced (+)-valencene was in agreement with that of the authentic standard.
GC–MS analysis of the extracted n-dodecane phase of shake-flask cultures of strain V02. a The GC–MS profiles of products produced by V02 strain and (+)-valencene standard. b Mass spectrum of (+)-valencene (peak 3). Peak 1 and peak 2 were identified as n-tridecane and n-tetradecane, which could be the contaminants from the used dissolvent n-dodecane. The GC–MS profiles of solvent control and the obtained MS–MS profiles of n-tridecane and n-tetradecane were included in the Additional file 1: Fig. S2
Engineering the MVA pathway to improve (+)-valencene production
To further improve (+)-valencene production, FPP synthase ERG20 that catalyzes the condensation of IPP and its isomer DMAPP into GPP and consecutively the incorporation of GPP with one extra IPP to form FPP [1], was first overexpressed in V04 to increase the metabolic flux to FPP. Considering that FPP is also the substrate for squalene and farnesol synthesis, we tested the effect of expressing the fused form of ERG20 with CnVS using three different flexible linkers, namely GSG, GGGGS, and GSGGGGS, on (+)-valencene formation with an expectation to minimize the competitive FPP consumption by channeling the metabolic flux to the target product [24,Plasmid and strain construction Phusions High Fidelity DNA polymerase (Thermo Fisher Scientific, Germany) was used for gene amplification according to the recommended protocol. Ligations in the present study were performed with T4 DNA ligase or T5 exonuclease-dependent assembly [46]. The DNA sequence of C. nootkatensis CnVS (Genbank accession: JC245925.1) was codon optimized and synthesized (Tsingke, Qingdao, China). The PGAL1 promoter, the CnVS gene, and the CYC1 terminator were orderly inserted in the pRS305 plasmid at SmaI and SacI sites to generate the pRS305-CnVS plasmid. The ERG20 and CnVS fusion gene was constructed by inserting the GSG, GGGGS, or GSGGGGS linker between these two genes with nested PCR. The PGAL1 promoter, the ERG20 and CnVS coding sequences, and the CYC1 terminator were assembled by T5 exonuclease-dependent assembly, and inserted at XhoI and SacI sites of pRS305 to obtain the pRS305-E3C, pRS305-C3E, pRS305-E5C, pRS305-E7C plasmid, respectively. tHMG1 was amplified from the genomic DNA of S. cerevisiae W303-1A and assembled between the PTPI1 promoter and the TCYC1 terminator, which was then inserted between BamHI and XhoI sites of pRS306 to obtain the pRS306-tHMG1 plasmid. To replace the endogenous promoter of ERG9 with the PHTX1 promoter, an integration cassette was constructed by assembling the upstream homologous region of the PERG9 promoter (70 bp), loxP-kanMX-loxP, PHTX1, and the downstream homologous region of PERG9 promoter (70 bp) in the desired order. The H. muticus HPO (EF569601.1) mutant (V482I A484I) gene and the A. thaliana atr1 (NM_118585.3) were codon optimized and synthesized by Tsingke. Their expression cassette were then separately constructed under the control of the PGAL1 promoter and the TCYC1 terminator by inserting the corresponding sequences adjacently in pRS304 between Eco53KI and SpeI sites to obtain the pRS304-HPO-ATR1 plasmid. Similarly, the codon optimized ValOx gene (HF913621.1) of P. sapidus was assembled between the PGAL1 promoter and the TCYC1 terminator, and the resulted expression cassette was inserted in pRS304 between Eco53KI and SpeI sites to obtain the pRS304-ValOx plasmid. The coding sequences for dehydrogenases ADH2 (Genbank: NM_001182812.1) and ADH6 (NM_001182831.3) of S. cerevisiae, ADH-C3 (XM_002492172) of P. pastoris, ZSD1 (AB480831.1) of Z. zerumbet, and ABA2 (HM036684.1) of C. sinensis were codon optimized and overexpressed under the control of the PGAL1 promoter and TCYC1 terminator, which were inserted between EcoRI and SacI sites in pRS423, respectively. All the plasmids constructed were verified by DNA sequencing (Tsingke, Qingdao, China). The strains and the corresponding plasmids used for their construction are listed in Table 2. Yeast transformations were performed by the standard lithium acetate method. Various metabolic engineered strains were thus constructed by the transformation of the appropriate plasmid into the S. cerevisiae W303-1A strain. The replacement of ERG9 promoter was accomplished by transforming the PCR amplified integration cassette into the appropriate strains. Transformants were selected on YNB auxotroph plates or selected for geneticin resistance. The correct integration was confirmed by PCR. A pre-culture was prepared by inoculating a single colony into 5 ml auxotroph YNB medium and cultivating O/N at 30 °C, 200 rpm. YPD medium with 0.2% glucose and 2% galactose as carbon sources was used as the following fermentation medium for (+)-valencene and (+)-nootkatone production. The flask fermentations were initiated by inoculating the pre-culture into 50 ml fermentation medium at an OD600nm of 0.01 and cultured at 30 °C, 200 rpm for 90 h. An organic layer of n-dodecane (10%, v/v) was added to extract products from the fermentation medium. The cultures were centrifuged at 8000 rpm for 10 min. The n-dodecane phase was sampled and stored at 4 °C for further analysis. Samples (500 μl) were mixed with 1 ml of n-hexane and 1 μl of the mixed sample was injected to be analyzed by GC-FID or GC–MS on a QP2010 instrument (Shimadzu). The production of (+)-valencene was analyzed using an rtx-1 column. The initial temperature was maintained at 40 °C for 2 min and the temperature program of 40–160 °C at 10 °C/min and 160–250 °C at 15 °C/min were followed. The production of β-nootkatol and (+)-nootkatone was analyzed using an rtx-1701 column and a temperature program of 70–200 °C at 10 °C/min and 200–280 °C at 30 °C/min. The produced farnesol was analyzed using an rtx-1701 column and a temperature program of 40–180 °C at 25 °C/min and 180–250 °C at 15 °C/min. The MS spectra of valencene, β-nootkatol, (+)-nootkatone, and farnesol were compared with those of authentic standards. The amounts of valencene, (+)-nootkatone, and farnesol were determined by GC-FID with the calibration curve generated by their respective standards. Due to the lack of standard, the amount of β-nootkatol in different strains was represented with their peak surface areas or their relative percentages. Due to the high volatility and low solubility of (+)-valencene, it is easily extracted by the added n-dodecane and thus the intracellular (+)-valencene concentration becomes very low, which is unfavorable for further (+)-nootkatone synthesis. In order to increase the solubility of (+)-valencene in aqueous phase, different concentrations of DMSO (1%, 2%, 4%, and 8%, v/v) and Triton X-100 (0.02%, 0.06%, and 0.1%, v/v) were added to the fermentation medium with 1/10 of n-dodecane. The growth of N06 strain was determined by measuring OD600nm. The production of (+)-nootkatone in the n-dodecane phase were measured by GC-FID analysis. The growth of metabolic engineered S. cerevisiae strains was determined by measuring the optical density at OD600nm. The pre-cultures of S. cerevisiae strains were inoculated in 50 ml YPD with different concentrations of n-dodecane and (+)-valencene at OD600nm of 0.1 and their growth was monitored by measuring the optical density at OD600nm for 28 h. The cells were harvested by centrifugation and washed two times with water. The dry cell weight (DCW) was obtained by measuring the weight after freeze drying.Flask fermentation for (+)-valencene and (+)-nootkatone production
Product analysis by GC-FID and GC–MS
Effects of DMSO and Triton X-100 on the production of (+)-nootkatone
Growth assay and the effect of n-dodecane or (+)-valencene on growth
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional files.
Abbreviations
- CPR:
-
Cytochrome P450 reductase
- DMAPP:
-
Dimethylallyl pyrophosphate
- ERG9:
-
Squalene synthase enzyme
- ERG20:
-
Diphosphate synthase
- FPP:
-
Farnesyl diphosphate
- GPP:
-
Geranyl pyrophosphate
- HPO:
-
Hyoscyamus muticus premnaspirodiene oxygenase
- IPP:
-
Isopentenyl diphosphate
- MEP:
-
Methyl-d-erythritol phosphate pathway
- MVA:
-
Mevalonate pathway
- SDR:
-
Short-chain dehydrogenase/reductase
- tHMG1:
-
Truncated form of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase
References
Paramasivan K, Mutturi S. Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit Rev Biotechnol. 2017;37:974–89.
Emmerstorfer-Augustin A, Pichler H. Production of aromatic plant terpenoids in recombinant baker’s yeast. Methods Mol Biol. 2016;1405:79–89.
Fraatz MA, Berger RG, Zorn H. Nootkatone-a biotechnological challenge. Appl Microbiol Biotechnol. 2009;83:35–41.
Leonhardt RH, Berger RG. Nootkatone. Biotechnol Isoprenoids. 2015;148:391–404.
Garcia-Cabeza AL, Marin-Barrios R, Moreno-Dorado FJ, Ortega MJ, Massanet GM, Guerra FM. Allylic oxidation of alkenes catalyzed by a copper-aluminum mixed oxide. Org Lett. 2014;16:1598–601.
Salvador JAR, Clark JH. The allylic oxidation of unsaturated steroids by tert-butyl hydroperoxide using surface functionalised silica supported metal catalysts. Green Chem. 2002;4:352–6.
Bloch K. Sterol molecule-structure, biosynthesis, and function. Steroids. 1992;57:378–83.
Maury J, Asadollahi MA, Moller K, Clark A, Nielsen J. Microbial isoprenoid production: an example of green chemistry through metabolic engineering. Adv Biochem Eng Biot. 2005;100:19–51.
Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria—a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295:517–24.
Gavira C, Hofer R, Lesot A, Lambert F, Zucca J, Werck-Reichhart D. Challenges and pitfalls of P450-dependent (+)-valencene bioconversion by Saccharomyces cerevisiae. Metab Eng. 2013;18:25–35.
Lucker J, Bowen P, Bohlmann J. Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (−)-germacrene d synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry. 2004;65:2649–59.
Sharon-Asa L, Shalit M, Frydman A, Bar E, Holland D, Or E, Lavi U, Lewinsohn E, Eyal Y. Citrus fruit flavor and aroma biosynthesis: isolation, functional characterization, and developmental regulation of Cstps1, a key gene in the production of the sesquiterpene aroma compound valencene. Plant J. 2003;36:664–74.
Beekwilder J, van Houwelingen A, Cankar K, van Dijk ADJ, de Jong RM, Stoopen G, Bouwmeester H, Achkar J, Sonke T, Bosch D. Valencene synthase from the heartwood of Nootka cypress (Callitropsis nootkatensis) for biotechnological production of valencene. Plant Biotechnol J. 2014;12:174–82.
Asadollahi MA, Maury J, Moller K, Nielsen KF, Schalk M, Clark A, Nielsen J. Production of plant sesquiterpenes in Saccharomyces cerevisiae: effect of ERG9 repression on sesquiterpene biosynthesis. Biotechnol Bioeng. 2008;99:666–77.
Frohwitter J, Heider SAE, Peters-Wendisch P, Beekwilder J, Wendisch VF. Production of the sesquiterpene (+)-valencene by metabolically engineered Corynebacterium glutamicum. J Biotechnol. 2014;191:205–13.
Scholtmeijer K, Cankar K, Beekwilder J, Wosten HAB, Lugones LG, Bosch D. Production of (+)-valencene in the mushroom-forming fungus S. commune. Appl Microbiol Biotechnol. 2014;98:5059–68.
Girhard M, Machida K, Itoh M, Schmid RD, Arisawa A, Urlacher VB. Regioselective biooxidation of (+)-valencene by recombinant E. coli expressing CYP109B1 from Bacillus subtilis in a two-liquid-phase system. Microb Cell Fact. 2009;8:36.
Cankar K, van Houwelingen A, Bosch D, Sonke T, Bouwmeester H, Beekwilder J. A chicory cytochrome P450 mono-oxygenase CYP71AV8 for the oxidation of (+)-valencene. FEBS Lett. 2011;585:178–82.
Takahashi S, Yeo YS, Zhao YX, O’Maille PE, Greenhagen BT, Noel JP, Coates RM, Chappell J. Functional characterization of premnaspirodiene oxygenase, a cytochrome P450 catalyzing regio- and stereo-specific hydroxylations of diverse sesquiterpene substrates. J Biol Chem. 2007;282:31744–54.
Cankar K, van Houwelingen A, Goedbloed M, Renirie R, de Jong RM, Bouwmeester H, Bosch D, Sonke T, Beekwilder J. Valencene oxidase CYP706M1 from Alaska cedar (Callitropsis nootkatensis). FEBS Lett. 2014;588:1001–7.
Fraatz MA, Riemer SJL, Stober R, Kaspera R, Nimtz M, Berger RG, Zorn H. A novel oxygenase from Pleurotus sapidus transforms valencene to nootkatone. J Mol Catal B-Enzym. 2009;61:202–7.
Krugener S, Krings U, Zorn H, Berger RG. A dioxygenase of Pleurotus sapidus transforms (+)-valencene regio-specifically to (+)-nootkatone via a stereo-specific allylic hydroperoxidation. Bioresour Technol. 2010;101:457–62.
Wriessnegger T, Augustin P, Engleder M, Leitner E, Muller M, Kaluzna I, Schurmann M, Mink D, Zellnig G, Schwab H, Pichler H. Production of the sesquiterpenoid (+)-nootkatone by metabolic engineering of Pichia pastoris. Metab Eng. 2014;24:18–29.
Zhao JZ, Bao XM, Li C, Shen Y, Hou J. Improving monoterpene geraniol production through geranyl diphosphate synthesis regulation in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2016;100:4561–71.
Jiang GZ, Yao MD, Wang Y, Zhou L, Song TQ, Liu H, **ao WH, Yuan YJ. Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae. Metab Eng. 2017;41:57–66.
Albertsen L, Chen Y, Bach LS, Rattleff S, Maury J, Brix S, Nielsen J, Mortensen UH. Diversion of flux toward sesquiterpene production in Saccharomyces cerevisiae by fusion of host and heterologous enzymes. Appl Environ Microbiol. 2011;77:1033–40.
Donald KAG, Hampton RY, Fritz IB. Effects of overproduction of the catalytic domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase on squalene synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol. 1997;63:3341–4.
Wang F, Lv XM, Xei WP, Zhou PP, Zhu YQ, Yao Z, Yang CC, Yang XH, Ye LD, Yu HW. Combining Gal4p-mediated expression enhancement and directed evolution of isoprene synthase to improve isoprene production in Saccharomyces cerevisiae. Metab Eng. 2017;39:257–66.
Withers ST, Gottlieb SS, Lieu B, Newman JD, Keasling JD. Identification of isopentenol biosynthetic genes from Bacillus subtilis by a screening method based on isoprenoid precursor toxicity. Appl Environ Microbiol. 2007;73:6277–83.
Asadollahi MA, Maury J, Schalk M, Clark A, Nielsen J. Enhancement of farnesyl fiphosphate pool as direct precursor of sesquiterpenes through metabolic engineering of the mevalonate pathway in Saccharomyces cerevisiae. Biotechnol Bioeng. 2010;106:86–96.
Cankar K, Jongedijk E, Klompmaker M, Majdic T, Mumm R, Bouwmeester H, Bosch D, Beekwilder J. (+)-Valencene production in Nicotiana benthamiana is increased by down-regulation of competing pathways. Biotechnol J. 2015;10:180–9.
Faulkner A, Chen XM, Rush J, Horazdovsky B, Waechter CJ, Carman GM, Sternweis PC. The LPP1 and DPP1 gene products account for most of the isoprenoid phosphate phosphatase activities in Saccharomyces cerevisiae. J Biol Chem. 1999;274:14831–7.
Scalcinati G, Knuf C, Partow S, Chen Y, Maury J, Schalk M, Daviet L, Nielsen J, Siewers V. Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquitepene alpha-santalene in a fed-batch mode. Metab Eng. 2012;14:91–103.
de Smidt O, du Preez JC, Albertyn J. The alcohol dehydrogenases of Saccharomyces cerevisiae: a comprehensive review. FEMS Yeast Res. 2008;8:967–78.
Gonzalez-Guzman M, Apostolova N, Belles JM, Barrero JM, Piqueras P, Ponce MR, Micol JL, Serrano R, Rodriguez PL. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell. 2002;14:1833–46.
Okamoto S, Yu FN, Harada H, Okajima T, Hattan J, Misawa N, Utsumi R. A short-chain dehydrogenase involved in terpene metabolism from Zingiber zerumbet. FEBS J. 2011;278:2892–900.
Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528.
Palmerin-Carreno DM, Castillo-Araiza CO, Rutiaga-Quinones OM, Calvo JRV, Trejo-Aguilar GM, Dutta A, Huerta-Ochoa S. Whole cell bioconversion of (+)-valencene to (+)-nootkatone by Yarrowia lipolytica using a three phase partitioning bioreactor. J Chem Technol Biotechnol. 2016;91:1164–72.
Kaspera R, Krings U, Nanzad T, Berger RG. Bioconversion of (+)-valencene in submerged cultures of the ascomycete Chaetomium globosum. Appl Microbiol Biotechnol. 2005;67:477–83.
Roth S, Kilgore MB, Kutchan TM, Muller M. Exploiting the catalytic diversity of short-chain dehydrogenases/reductases: versatile enzymes from plants with extended imine substrate scope. ChemBioChem. 2018;19:1849–52.
Davis EM, Ringer KL, McConkey ME, Croteau R. Monoterpene metabolism: cloning, expression, and characterization of menthone reductases from peppermint. Plant Physiol. 2005;137:873–81.
Ringer KL, Davis EM, Croteau R. Monoterpene metabolism: cloning, expression, and characterization of (−)-isopiperitenol/(−)-carveol dehydrogenase of peppermint and spearmint. Plant Physiol. 2005;137:863–72.
Trenchard IJ, Smolke CD. Engineering strategies for the fermentative production of plant alkaloids in yeast. Metab Eng. 2015;30:96–104.
Zhang W, Du L, Li FW, Zhang XW, Qu ZP, Hang L, Li Z, Sun JR, Qi FX, Yao QP, et al. Mechanistic insights into interactions between bacterial class I P450 enzymes and redox partners. ACS Catalysis. 2018;8:9992–10003.
Rimal H, Lee SW, Lee JH, Oh TJ. Understanding of real alternative redox partner of Streptomyces peucetius DoxA: prediction and validation using in silico and in vitro analyses. Arch Biochem Biophys. 2015;585:64–74.
**a YZ, Li K, Li JJ, Wang TQ, Gu LC, Xun LY. T5 exonuclease-dependent assembly offers a low-cost method for efficient cloning and site-directed mutagenesis. Nucleic Acids Res. 2019;47:e15.
Acknowledgements
We are grateful to Professor Shan Cen and Dr. Quanjie Li (Chinese Academy of Medical Sciences) for their assistance in the binding analysis of β-nootkatol and solavetivol in HPO by docking study and to Dr. Yu Shen for technical suggestions.
Funding
This work is supported by National Key R&D Program of China (No.2019YFA0905700), National Natural Science Foundation of China (31800047, 31970071), Shandong Provincial Natural Science Foundation (ZR2018BC006), China Postdoctoral Science Foundation (2017M622186), Shandong Technology Innovation Center of Synthetic Biology (sdsynbio-2018PY-01),Young Scholars Program of Shandong University and the Fundamental Research Funds of Shandong University (2017TB0010).
Author information
Authors and Affiliations
Contributions
WL, XM and HL designed this study; HL and XM performed the experiments; all the author analyzed the data; XM and WL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Additional figures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Meng, X., Liu, H., Xu, W. et al. Metabolic engineering Saccharomyces cerevisiae for de novo production of the sesquiterpenoid (+)-nootkatone. Microb Cell Fact 19, 21 (2020). https://doi.org/10.1186/s12934-020-1295-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-020-1295-6