Abstract
Background
The most recent treatment guidelines for type 2 diabetes (T2D) recommend sodium-glucose cotransporter 2 (SGLT2) inhibitors should be considered preferentially in patients with T2D with either a high cardiovascular risk or with cardiovascular disease (CVD), regardless of their diabetes status and prior use of conventional metformin therapy. Whether the therapeutic impact of SGLT2 inhibitors on clinical parameters differs according to the use of metformin therapy however remains unclear.
Methods
The study was a post hoc analysis of the EMBLEM trial (UMIN000024502). All participants (n = 105; women 31.4%; mean age 64.8 years) had both T2D and CVD and were randomized to either 24 weeks of empagliflozin 10 mg daily or placebo. Analysis of the data assessed the effect of empagliflozin on changes from baseline to 24 weeks in glycemic and non-glycemic clinical parameters, according to the baseline use of metformin.
Results
Overall, 53 (50.5%) patients received baseline metformin. In the 52 patients treated with empagliflozin (48.1% with baseline metformin), the decrease in systolic blood pressure from baseline levels was greater in patients receiving metformin, compared to that observed in metformin-naïve patients (group difference − 8.5 [95% confidence interval (CI) − 17.7 to 0.6 mmHg], p = 0.066). Reduction in body mass index (BMI) was significantly greater in patients receiving baseline metformin, relative to nonusers (− 0.54 [95% CI − 1.07 to − 0.01] kg/m2, p = 0.047). The group ratio (baseline metformin users vs. nonusers) of proportional changes in the geometric mean of high-sensitivity Troponin-I (hs-TnI) was 0.74 (95% CI 0.59 to 0.92, p = 0.009). No obvious differences were observed in glycemic parameters (fasting plasma glucose, glycohemoglobin, and glycoalbumin) between the baseline metformin users and nonusers.
Conclusion
Our findings suggest 24 weeks of empagliflozin treatment was associated with an improvement in glycemic control, irrespective of the baseline use of metformin therapy. The effects of empagliflozin on reductions in BMI and hs-TnI were more apparent in patients who received baseline metformin therapy, compared to that observed in metformin-naïve patients.
Trial registration University Medical Information Network Clinical Trial Registry, number 000024502
Similar content being viewed by others
Introduction
Based on accumulated clinical evidence and demonstration of safety and efficacy, metformin has become recognized as an established glucose-lowering agent for the initial treatment of type 2 diabetes (T2D) [1]. In the latest European Society of Cardiology (ESC) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the European Association for the Study of Diabetes (EASD), it was also recommended that metformin should be considered as first-line therapy in patients with T2D, especially in overweight patients without cardiovascular disease (CVD) and those with a moderate cardiovascular risk [2]. Recent cardiovascular outcome trials (CVOTs) on several classes of glucose-lowering agents, including sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists, have shown that these agents reduced cardiovascular events and death in patients with T2D at high cardiovascular risk or with established CVD. This led to a recent groundbreaking revision of treatment guidelines to use either agents in these patients, independent of baseline use of metformin [1, 2]. Given the previous position of metformin in diabetes care, this was likely a marked paradigm shift in the selection of glucose-lowering agents.
In the CVOTs completed before the development of such a paradigm shift, almost all participants (i.e., more than two-thirds) received baseline metformin therapy in accordance with conventional treatment strategy for diabetes care and had the agents investigated in this study added to metformin therapy [3,4,5,6,7,8,9,10,11,12]. This raised the clinical question as to whether or not the cardiovascular benefits of the agents would be expected even in patients with T2D at high cardiovascular risk or those with CVD, independent of the effects of baseline metformin. However, subsequent analyses demonstrated that treatment with these agents consistently improved cardiovascular outcomes regardless of the baseline use of metformin [13,14,15,16,17]. These findings also support recent updated treatment guidelines for better outcomes especially in patients with T2D either at high cardiovascular risk or with CVD. However, only limited clinical data are currently available regarding differences in the effect of these agents on glycemic and non-glycemic parameters in this patient population. Identifying these differences may help to achieve optimal selection of glucose-lowering agents for secondary prevention of CVD in actual clinical settings.
The EMBLEM (Effect of Empagliflozin on Endothelial Function in Cardiovascular High Risk Diabetes Mellitus: Multi-Center Placebo-Controlled Double-Blind Randomized) trial had the primary aim of determining whether 24 weeks of empagliflozin treatment affected peripheral endothelial function in patients with T2D and established CVD [18, 19]. In that trial, about one-half of the participants did not receive metformin therapy at baseline. Therefore, the current post hoc analysis of the EMBLEM trial examined whether the effect of 24 weeks of empagliflozin treatment on glycemic and non-glycemic clinical parameters in patients with T2D and CVD differed according to the use of baseline metformin treatment.
Methods
Study design
The EMBLEM trial (UMIN000024502) was an investigator-initiated, multi-center, placebo-controlled, double-blinded, randomized-controlled trial undertaken in 16 centers in Japan. The details of the study design and primary results have been reported previously [18,19,20]. Briefly, eligible patients were ≥ 20 years old, with T2D, and a glycohemoglobin (HbA1c) level ranging from 6.0 and 10.0%, who were clinically stable without changes in T2D therapy for at least one month before consent, and had a previous history of at least one established CVD (coronary artery disease, stroke, peripheral artery disease, presence of known coronary artery stenosis (≥ 50%), or heart failure (HF) with a New York Heart Association classification I to III and clinically stable by the use of HF-medications for at least one month before consent). Key exclusion criteria were type 1 diabetes, a history of diabetic ketoacidosis or diabetic coma within the last 6 months, severe renal dysfunction (estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2 or undergoing dialysis), serious liver dysfunction, a history of atherosclerotic CVD within 3 months before consent, and prior use of a SGLT2 inhibitor within one month before consent.
The participants were assigned randomly to either 10 mg of daily empagliflozin or to placebo, using a web-based minimization system that balances for HbA1c (< 7.0 or ≥ 7.0%), age (< 65 or ≥ 65 years), systolic blood pressure (BP) (< 140 or ≥ 140 mmHg), and current smoking status at the time of screening. The participants underwent scheduled visits after 4, 12, and 24 weeks for dispensing of drugs and assessment of study endpoints. Although no specific goal of glycemic control was set in the EMBLEM trial, all participants were to be treated in accordance with the local treatment guidelines for T2D of the Japan Diabetes Society at that time. Each participant’s background medications, including glucose-lowering therapy, were in principle unchanged during the trial. However, if the therapeutic effect of the medications was insufficient, the addition of glucose-lowering agents other than SGLT2 inhibitors or an increased dosage of background medications were allowed at the judgement of the local investigator.
The ethical committees of the participating institutions approved the study protocol. Written, informed consent for participation in the study was obtained from all the subjects. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Outcome measures
The details of the original outcome measures in the EMBLEM trial have been reported previously [20]. The main endpoints in the post hoc analysis using data obtained from the trial were changes from baseline to week 24 in glycemic parameters (fasting plasma glucose [FPG], HbA1c, and glycoalbumin [GA]) and non-glycemic parameters, including systolic and diastolic BP, heart rate (HR), double product (systolic BP × HR), body mass index (BMI), N-terminal pro-brain natriuretic peptide (NT-proBNP), and high-sensitivity Troponin-I (hs-TnI). Of the laboratory markers, the assays of GA (SRL, Inc., Tokyo, Japan), NT-proBNP (SRL, Inc., Tokyo, Japan), and hs-TnI (Abbott Japan LLC, Tokyo, Japan) were performed at central laboratories. The post hoc analysis assessed the effect of empagliflozin on these variables according to the use or nonuse of baseline metformin.
Statistical analysis
All the analyses were conducted on the full analysis set, which included all participants who had received at least one dose of the study medication after randomization and who did not have any serious violation of the protocol. Baseline demographics and characteristics were expressed as numbers (percentages) for categorical variables and as means ± standard deviation for continuous variables. Data on NT-proBNP and hs-TnI were expressed as geometric mean (95% confidence interval [CI]), and the proportional changes from baseline to week 24 calculated based on a natural logarithmic scale. Inter-group differences and ratios were compared using Welch’s t tests for continuous variables or Fisher’s exact test for categorical variables. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). A two-sided significance level of p < 0.05 was used for all assessments, with no adjustment for multiplicity being used in the analyses.
Results
Baseline characteristics
A detailed participant flow-chart and the overall baseline characteristics of the EMBLEM trial have been reported elsewhere [18, 19]. Briefly, of the 117 patients randomized, 105 patients (64.8 ± 10.4 years old; women 33 [31.4%]; HbA1c 7.2 ± 0.8%; diabetes duration 13.3 ± 11.1 years) were included in the full analysis set (empagliflozin group n = 52, placebo group n = 53). Overall, 69 (65.7%), 18 (17.1%), and 79 (75.2%) patients were taking an angiotensin-converting enzyme or angiotensin receptor blocker, diuretic, and statin therapy, respectively. The majority of patients (69.5%) were taking a dipeptidyl peptidase-4 (DPP-4) inhibitor. Twenty-four patients (22.9%) were taking one type of glucose-lowering medication, while 67 patients (63.8%) were taking ≥ 2 types of these medications.
At baseline, a total of 53 (50.5%) patients were receiving metformin therapy, with 25 (48.1%) in the empagliflozin group and 28 (52.8%) in the placebo group. As shown in Table 1, the baseline characteristics were almost similar between the randomization groups, irrespective of the use of metformin at baseline.
Changes in clinical parameters according to baseline metformin use
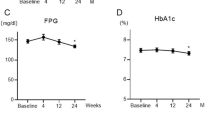
Changes from baseline to week 24 in clinical parameters according to the baseline use of metformin and randomization group are shown in Table 2. In patients receiving baseline metformin, the magnitude of reduction in the levels of systolic BP, double product, BMI, HbA1c, and GA were greater in patients treated with 24 weeks of empagliflozin, compared to those on placebo. In contrast, examination of the magnitude of changes in clinical parameters in metformin-naïve patients at baseline showed these were similar in the randomization groups, except for FPG. Irrespective of the use of baseline metformin, no significant randomization-based group ratios of proportional changes in NT-proBNP (metformin users, 1.11 [95% CI 0.78 to 1.58], p = 0.551; nonusers, 1.11 [95% CI 0.76 to 1.64], p = 0.579) and hs-TnI (metformin users, 0.93 [95% CI 0.74 to 1.16], p = 0.490; nonusers, 0.96 [95% CI 0.73 to 1.27], p = 0.788) were observed between the randomization groups.
Effect of empagliflozin in metformin users and nonusers
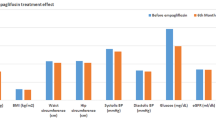
In the group treated with empagliflozin, the changes in systolic BP and double product from baseline to week 24 were greater in patients receiving baseline metformin therapy than those in metformin-naïve patients. However, these differences were not statistically significant (Fig. 1A, D). The reductions in BMI were significantly greater in patients who received baseline metformin therapy, compared to those who did not (Fig. 1E). No significant differences were observed between baseline metformin users and nonusers for the effect of empagliflozin treatment on the other parameters; HR, diastolic BP, and glycemic parameters (FPG, HbA1c, and GA) (Fig. 1B, C, F–H). The effect of empagliflozin on NT-proBNP concentration was also similar between the baseline metformin users and nonusers (Fig. 1I), while the group ratio (baseline metformin users vs. nonusers) of proportional changes in the geometric mean of hs-TnI was 0.74 (95% CI 0.59 to 0.92, p = 0.009: Fig. 1J).
Change in clinical parameters from baseline to week 24 in patients treated with empagliflozin according to baseline use of metformin. Blue (red) indicates the group with (without) baseline metformin therapy. Values located at the bottom of each panel indicate the mean group difference (95% confidence interval) in the magnitude of change from baseline to week 24 in systolic blood pressure (A), diastolic blood pressure (B), heart rate (C), double product (systolic blood pressure × heart rate: D body mass index (E), fasting plasma glucose (F), glycohemoglobin (G), and glycoalbumin (H) or the mean group ratio (95% confidence interval) of the change ratio from baseline to 24 weeks in the geometric means of NT-proBNP (I) and hs-TnI (J). hs-TnI, high-sensitivity Troponin-I; NT-proBNP, N-terminal pro-brain natriuretic peptide
Discussion
This post hoc analysis of the EMBLEM trial on patients with T2D and established CVD, showed that the effects of 24 weeks of empagliflozin treatment on non-glycemic parameters, such as BMI and hs-TnI level, were more apparent in patients who had received baseline metformin therapy compared to metformin-naïve patients. In contrast, the impact of empagliflozin treatment on standard glycemic parameters was similar in patients who had received baseline metformin therapy to those who had not. To our knowledge, this is the first study in patients with T2D and CVD that assessed whether the effects of empagliflozin on clinical parameters differ according to the use of baseline metformin. Given the recent recommendation for SGLT2 inhibitors to be considered as key drugs for improving outcomes in patients with T2D at high cardiovascular risk or with CVD, regardless of previous metformin use [1, 2], the findings of our study may provide clinicians with novel insights on the effects of empagliflozin on a range of clinical parameters used frequently in the daily care of diabetes and cardiovascular disease, according to the patient’s prior use of metformin.
In the landmark trial of the United Kingdom Prospective Diabetes Study (UKPDS), metformin, relative to diet alone or other glucose-lowering agents such as sulfonylureas and insulin, was shown to reduce the risk of cardiovascular events and mortality in obese patients with newly diagnosed T2D [21]. The study also demonstrated that this early treatment effect was maintained, as a legacy effect, for as long as 10 years after the study intervention [22]. Based on its high degree of safety and efficacy and low-cost, metformin has been recommended over two decades as the first-line glucose-lowering agent for treating T2D. However, this recommendation was made before the use of modern and established cardiovascular protective drugs, such as statins and renin-angiotensin aldosterone system inhibitors. Accordingly, the majority of participants in earlier and recent CVOTs on newer glucose-lowering agents, including SGLT2 inhibitors, were administered metformin at baseline. Nonetheless, previous meta-analyses have reported heterogeneous and controversial effects of metformin on CVD and mortality, due mainly to a lack of good evidence obtained using current standards of care and trial methodology [23,36]. These results might indicate that the baseline use of metformin helps to enhance and retain the immediate BW-reducing effect of SGLT2 inhibitors in the present study. Furthermore, combination therapy of a SGLT2 inhibitor and metformin might have efficiently promoted BW loss through intrinsic insulin saving [37, 38], selective fat mass reduction [39, 40], and mitigation of compensatory overeating induced by chronic administration of SGLT2 inhibitors [41, 42]. Collectively, this combination therapy may provide synergistic benefits in some non-glycemic parameters, and as a consequence deliver a comprehensive therapeutic approach for diabetes-related complications, beyond that provided by lowering of glucose levels.
Interestingly, our analysis of established markers of cardiac stress and damage showed that the baseline levels of NT-proBNP and hs-TnI in patients who had received baseline metformin appeared to be lower than those in nonusers, although this difference was not statistically significant. The prevalence of a previous history of HF was similar between patients with or without baseline metformin therapy. This finding that the use of baseline metformin therapy might be associated with lower levels of these cardiac markers may, in part, support the possibility that metformin has robust cardioprotective effects via multifaceted molecular mechanisms, such as AMP-activated protein kinase-dependent and -independent signaling pathways [43]. In fact, the latest guidelines still recommend metformin therapy should be continued or included in patients with T2D and HF [1, 2]. Furthermore, empagliflozin treatment was associated with significantly greater reductions in hs-TnI concentrations in patients with baseline metformin therapy, compared to that observed in metformin-naïve patients. This finding raises the possibility that the protective effect of this combination therapy on micro-myocardial damage may, at least in part, be enhanced by several cardiometabolic actions, including a modulation of impaired cardiac insulin signaling [44]. However, the clinical impact of SGLT2 inhibitors on those cardiac biomarkers remains controversial [45]. Further research is therefore warranted in order to better understand which markers are most suitable for clinical monitoring of the cardiovascular effects of SGLT2 inhibitors.
The present study had several limitations. First, it was a post hoc analysis that used data obtained from the EMBLEM trial that was designed primarily to assess the effect of empagliflozin treatment, relative to placebo, on peripheral endothelial function. Second, although about one-half of the participants in the study were metformin-naïve at baseline, the clinical reasons why these patients did not receive metformin are unknown. Hence, some confounding factors with metformin use, such as renal function and severity of HF may have partially affected the impact of empagliflozin treatment on our study endpoints in the patients with or without baseline metformin therapy. Third, the small number of patients in the subgroups may not have provided sufficient statistical power to detect true group differences in the clinical parameters examined in the study. Furthermore, adjustments of potential confounding factors at baseline and changes in the glucose-lowering agents during the trial were not carried out due to the small sample size and limited clinical information. Finally, because the EMBLEM trial included only Japanese patients with T2D and CVD, who were clinically stable and met the study inclusion and exclusion criteria, further research is needed to assess whether the present findings are applicable to other ethnicities and/or different patient populations.
Conclusion
This study in patients with T2D and CVD demonstrated that 24 weeks of empagliflozin treatment was associated with an improvement in glycemic control, irrespective of baseline use of metformin therapy. The effects of empagliflozin on reductions in BMI and hs-TnI were more apparent in patients who received baseline metformin, compared to that observed in metformin-naïve patients. Based on the most recent guidelines for T2D, especially for patients with increased cardiorenal risk, the clinical opportunity to prescribe SGLT2 inhibitors will likely increase, and further research is therefore needed to investigate the effect of these drugs on clinical parameters, taking into account the background situation of conventional glucose-lowering agents, such as metformin.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request (tanakaa2@cc.saga-u.ac.jp).
Abbreviations
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- BW:
-
Body weight
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- CVOT:
-
Cardiovascular outcome trial
- DPP-4:
-
Dipeptidyl peptidase-4
- EASD:
-
European Association for the Study of Diabetes
- ESC:
-
European Society of Cardiology
- FPG:
-
Fasting plasma glucose
- GA:
-
Glycoalbumin
- HbA1c:
-
Glycohemoglobin
- HF:
-
Heart failure
- HR:
-
Heart rate
- hs-TnI:
-
High-sensitivity Troponin-I
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- SGLT2:
-
Sodium glucose co-transporter 2
References
Pharmacologic Approaches to Glycemic Treatment. Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111-s124.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, Lawson FC, ** L, Wei X, Lewis EF, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet (London, England). 2018;392(10157):1519–29.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New Engl J Med. 2019;8:224.
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Ryden L, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet (London, England). 2019;394(10193):121–30.
Crowley MJ, McGuire DK, Alexopoulos AS, Jensen TJ, Rasmussen S, Saevereid HA, Verma S, Buse JB. Effects of liraglutide on cardiovascular outcomes in type 2 diabetes patients with and without baseline metformin use: post hoc analyses of the LEADER Trial. Diabetes Care. 2020;43(9):e108–10.
Ferrannini G, Gerstein H, Colhoun HM, Dagenais GR, Diaz R, Dyal L, Lakshmanan M, Mellbin L, Probstfield J, Riddle MC, et al. Similar cardiovascular outcomes in patients with diabetes and established or high risk for coronary vascular disease treated with dulaglutide with and without baseline metformin. Eur Heart J. 2020;8:651.
Inzucchi SE, Fitchett D, Jurišić-Eržen D, Woo V, Hantel S, Janista C, Kaspers S, George JT, Zinman B. Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose-lowering therapy? Diabetes Obes Metab. 2020;22(4):631–9.
Neuen BL, Arnott C, Perkovic V, Figtree G, de Zeeuw D, Fulcher G, Jun M, Jardine MJ, Zoungas S, Pollock C, et al. Sodium-glucose co-transporter-2 inhibitors with and without metformin: a meta-analysis of cardiovascular, kidney and mortality outcomes. Diabetes Obes Metab. 2021;23(2):382–90.
Masson W, Lavalle-Cobo A, Lobo M, Masson G, Molinero G. Novel antidiabetic drugs and risk of cardiovascular events in patients without baseline metformin use: a meta-analysis. Eur J Prev Cardiol. 2021;28(1):69–75.
Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, Takamura T, Taguchi I, Hisauchi I, Toyoda S, et al. Secondary analyses to assess the profound effects of empagliflozin on endothelial function in patients with type 2 diabetes and established cardiovascular diseases: the placebo-controlled double-blind randomized effect of empagliflozin on endothelial function in cardiovascular high risk diabetes mellitus: Multi-center placebo-controlled double-blind randomized trial. J Diabetes Investig. 2020;11(6):1551–63.
Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, Takamura T, Taguchi I, Hisauchi I, Toyoda S, et al. Effect of empagliflozin on endothelial function in patients with type 2 diabetes and cardiovascular disease: results from the multicenter, randomized, placebo-controlled double-blind EMBLEM trial. Diabetes care. 2019;42(10):e159–61.
Tanaka A, Shimabukuro M, Okada Y, Taguchi I, Yamaoka-Tojo M, Tomiyama H, Teragawa H, Sugiyama S, Yoshida H, Sato Y, et al. Rationale and design of a multicenter placebo-controlled double-blind randomized trial to evaluate the effect of empagliflozin on endothelial function: the EMBLEM trial. Cardiovasc Diabetol. 2017;16(1):48.
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–65.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017;60(9):1620–9.
Han Y, **e H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol. 2019;18(1):96.
Zhang K, Yang W, Dai H, Deng Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: Results from meta-analysis. Diabetes Res Clin Pract. 2020;160:108001.
The Japan Diabetes Society. Treatment guide for diabetes 2018–2019. BUNKODO; 2018.
Araki E, Tanaka A, Inagaki N, Ito H, Ueki K, Murohara T, Imai K, Sata M, Sugiyama T, Ishii H, et al. Diagnosis, prevention, and treatment of cardiovascular diseases in people with type 2 diabetes and prediabetes—a consensus statement jointly from the Japanese Circulation Society and the Japan Diabetes Society. Circ J. 2020;85(1):82–125.
Araki E, Tanaka A, Inagaki N, Ito H, Ueki K, Murohara T, Imai K, Sata M, Sugiyama T, Ishii H, et al. Diagnosis, prevention, and treatment of cardiovascular diseases in people with type 2 diabetes and prediabetes: a consensus statement jointly from the Japanese Circulation Society and the Japan Diabetes Society. Diabetol Int. 2021;12(1):1–51.
Cho YK, Kang YM, Lee SE, Lee J, Park JY, Lee WJ, Kim YJ, Jung CH. Efficacy and safety of combination therapy with SGLT2 and DPP4 inhibitors in the treatment of type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2018;44(5):393–401.
Min SH, Yoon JH, Moon SJ, Hahn S, Cho YM. Combination of sodium-glucose cotransporter 2 inhibitor and dipeptidyl peptidase-4 inhibitor in type 2 diabetes: a systematic review with meta-analysis. Sci Rep. 2018;8(1):4466.
Ni L, Yuan C, Chen G, Zhang C, Wu X. SGLT2i: beyond the glucose-lowering effect. Cardiovasc Diabetol. 2020;19(1):98.
Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61(10):2098–107.
Tanaka A, Node K. Hypertension in diabetes care: emerging roles of recent hypoglycemic agents. Hypertens Res. 2021;3:114.
Lee PC, Ganguly S, Goh SY. Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obes Rev. 2018;19(12):1630–41.
Odawara M, Kawamori R, Tajima N, Iwamoto Y, Kageyama S, Yodo Y, Ueki F, Hotta N. Long-term treatment study of global standard dose metformin in Japanese patients with type 2 diabetes mellitus. Diabetol Int. 2017;8(3):286–95.
Apolzan JW, Venditti EM, Edelstein SL, Knowler WC, Dabelea D, Boyko EJ, Pi-Sunyer X, Kalyani RR, Franks PW, Srikanthan P, et al. Long-term weight loss with metformin or lifestyle intervention in the diabetes prevention program outcomes study. Ann Intern Med. 2019;170(10):682–90.
Koshizaka M, Ishikawa K, Ishibashi R, Maezawa Y, Sakamoto K, Uchida D, Nakamura S, Yamaga M, Yokoh H, Kobayashi A, et al. Comparing the effects of ipragliflozin versus metformin on visceral fat reduction and metabolic dysfunction in Japanese patients with type 2 diabetes treated with sitagliptin: a prospective, multicentre, open-label, blinded-endpoint, randomized controlled study (PRIME-V study). Diabetes Obes Metab. 2019;21(8):1990–5.
Han KA, Chon S, Chung CH, Lim S, Lee KW, Baik S, Jung CH, Kim DS, Park KS, Yoon KH, et al. Efficacy and safety of ipragliflozin as an add-on therapy to sitagliptin and metformin in Korean patients with inadequately controlled type 2 diabetes mellitus: A randomized controlled trial. Diabetes Obes Metab. 2018;20(10):2408–15.
Nagai Y, Fukuda H, Kawanabe S, Nakagawa T, Ohta A, Tanaka Y. differing effect of the sodium-glucose cotransporter 2 inhibitor ipragliflozin on the decrease of fat mass vs. lean mass in patients with or without metformin therapy. J Clin Med Res. 2019;11(4):297–300.
Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–31.
Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, Pelleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity (Silver Spring, Md). 2012;20(8):1645–52.
Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 Inhibition. Diabetes Care. 2015;38(9):1730–5.
Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20(6):953–66.
Zamora M, Villena JA. Contribution of impaired insulin signaling to the pathogenesis of diabetic cardiomyopathy. Int J Mol Sci. 2019;20:11.
Tanaka A, Node K. How should we monitor the cardiovascular benefit of sodium-glucose cotransporter 2 inhibition? Cardiovasc Diabetol. 2020;19(1):206.
Acknowledgements
The authors thank all the investigators, board members, coordinators, CRO (DOT WORLD CO., LTD. Tokyo, Japan) and patients who participated in the EMBLEM trial.
Funding
The work was funded by Boehringer Ingelheim and Eli Lilly and Company. The funders of the trial had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception, design, and operations. Data collection was performed by MS, HirokT, YO, TT, IT, ST, and HirofT. Funding acquisition and data analysis were performed by AT and KN. KN was a principal investigator in the EMBLEM trial. The first draft of the manuscript was written by AT and all authors reviewed previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The ethical committees of the participating institutions approved the study protocol. Written, informed consent for participation in the study was obtained from all the subjects. This trial was performed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Consent for publication
All authors have read and approved the submission of the manuscript. The manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language. If the manuscript is accepted, we approve it for publication in Cardiovascular Diabetology.
Competing interests
AT received honoraria from Boehringer Ingelheim and research funding from GlaxoSmithKline. MS received honorarium and an endowed chair from Boehringer Ingelheim. HirokT received lecture fees from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Kowa, Takeda, Mitsubishi Tanabe, and Sanwa Kagaku Kenkyusyo. YO received lecture fees from Astellas, AstraZeneca, MSD, Ono, Mitsubishi Tanabe, Bayer, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Daiichi Sankyo, Kissei, Novartis, Kowa, and Sanwa Kagaku Kenkyusho and research funds from Kowa and Mitsubishi Tanabe. TT received honoraria for lecturing from MSD, Sumitomo Dainippon, and Mitsubishi Tanabe, research funds from Kowa, Mitsubishi Tanabe, and Taisho, and scholarships from Astellas, Novo Nordisk, Ono, and Takeda. HirofT received research funding from Omron Health Care, Asahi Calpis Wellness, and Tei**. SU received research grants from Bristol-Myers Squibb and Kowa, non-purpose research grants from Bristol-Myers Squibb, Chugai, MSD, Pfizer, and Takeda and lecture fees from Boehringer Ingelheim and MSD. YH received consulting fees from Mitsubishi Tanabe related to this study, as well as honoraria and grants from Tei**, Boehringer Ingelheim, MSD, Sanofi, AstraZeneca, Kyowa Hakko Kirin, Takeda, Astellas, Daiichi Sankyo, Mochida, Nihon Kohden, Shionogi, Nippon Sigmax, Sanwa Kagaku Kenkyusho, Unex, and Kao and honoraria from Radiometer, Omron, Sumitomo Dainippon, Otsuka, Torii, Kowa, Fujiyakuhin, Amgen, Nippon Shinyaku, Itamar, Bayer, Eli Lilly, and Ono. KN received research grants from Asahi Kasei, Astellas, Bayer, Boehringer Ingelheim, Mitsubishi Tanabe, Tei**, and Terumo, scholarships from Astellas, Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Daiichi Sankyo Healthcare, Takeda, and Tei** and personal fees from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo Healthcare, Eli Lilly, Kowa, Mitsubishi Tanabe, MSD, Novartis, Ono, Takeda, and Tei**. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tanaka, A., Shimabukuro, M., Teragawa, H. et al. Comparison of the clinical effect of empagliflozin on glycemic and non-glycemic parameters in Japanese patients with type 2 diabetes and cardiovascular disease treated with or without baseline metformin. Cardiovasc Diabetol 20, 160 (2021). https://doi.org/10.1186/s12933-021-01352-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-021-01352-0