Abstract
Mechanosensitive Piezo ion channels were first reported in 2010 in a mouse neuroblastoma cell line, opening up a new field for studying the composition and function of eukaryotic mechanically activated channels. During the past decade, Piezo ion channels were identified in many species, such as bacteria, Drosophila, and mammals. In mammals, basic life activities, such as the sense of touch, proprioception, hearing, vascular development, and blood pressure regulation, depend on the activation of Piezo ion channels. Cumulative evidence suggests that Piezo ion channels play a major role in lung vascular development and function and diseases like pneumonia, pulmonary hypertension, apnea, and other lung-related diseases. In this review, we focused on studies that reported specific functions of Piezos in tissues and emphasized the physiological and pathological effects of their absence or functional mutations on the respiratory system.
Similar content being viewed by others
Introduction
Mechanotransduction is a fundamental physiological process that converts mechanical stimuli into bioelectrical and biochemical signals [1] and affects the cellular response to external changes and the growth and development of skeletal organs. Mechanical forces that stimulate cell membrane and intracellular signals rapidly activate mechanically activated (MA) ion channels [2]. Previous studies have identified ion channels in bacteria, moss [3], Drosophila [4], and mammals [5]. In bacteria, these channels respond to osmotic shock by permeating ions and osmolytes to prevent cell lysis [6]. In mammals, basic life activities, such as the sense of touch [7], proprioception [8], hearing [9], vascular development, and blood pressure regulation, depend on MA ion channels to initiate membrane depolarization in excitable cells and Ca2+ conduction in non-excitable cells [10,11,12]. Therefore, accurate mechanotransduction is essential for normal organ function, while abnormal or faulty mechanotransduction may lead to a variety of diseases.
The discovery of Piezos in 2010 opened up a new field for studying the composition and function of eukaryotic MA channels [10]. Piezo ion proteins have been identified as pore-forming subunits of non-selective cationic mechanosensitive ion channels, the expression of which is required for several processes involving mechanotransduction [13]. During the past decade, studies have indicated that Piezos are expressed in several tissues. Piezo1 ion channel is mainly expressed in non-excitable cells, such as endothelial cells (ECs) and adipocytes (Additional file 1: Figure S1). In contrast, the Piezo2 ion channel mainly exists in sensory neurons, such as dorsal root ganglia (DRG) [14], Merkel cells [15], skeletal tissues, bladder urethral epithelial cells [16], and enterochromaffin cells [17] (Additional file 1: Figure S2).
In addition, cumulative evidence suggests that Piezo ion channels play a major role in lung vascular development and function [18] and diseases like pneumonia [19], pulmonary hypertension (PH) [20], apnea [21], and other lung-related diseases. Changes in the mechanical properties and conditions of the airway and lungs, such as lung contraction and inflation, activate airway mechanical sensors [22]. Piezo, as a mechanosensitive ion protein, plays a crucial role in the pathophysiological response of the lung. Therefore, the current review aimed to briefly summarize the structure of Piezo ion channels and the diseases caused by deletion or mutation in Piezos in different tissues and organs, and then focus on discussing how Piezos might be involved in the physiology and pathophysiology of the respiratory system.
Discovery of Piezo1 and Piezo2
Bertrand Coste et al. were the first to identify that mechanosensitive Piezo ion channels rapidly respond to mechanical stimuli and mediate the influx of cations into cells to induce cellular excitation and signal transduction [10]. Bertrand Coste et al. identified Fam38A by selecting known cation channels and proteins of unknown function from the mouse neuroblastoma Neuro2A cell line and knocking out the genes one by one [10]. Since Fam38A encodes a protein required for stress-activated ion channel expression, it was named Piezo1, which means "pressure" in Greek. Piezo2 was later identified as a gene homologous to Piezo1 [2]. According to the NCBI database, only one Piezo member exists in plants, invertebrates, and Drosophila melanogaster but two in vertebrates [23]. Piezo is the largest transmembrane ion channel identified so far containing 114 transmembrane domains (TMs). Moreover, they show no sequence homology with known ion channels [24]. This groundbreaking finding has attracted the attention of several researchers worldwide.
Structure of Piezo1 and Piezo2
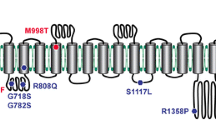
In the past decade, a large number of studies elaborated on the molecular structure of Piezos. The structural determination of Piezo1 and Piezo2 using cryo-electron microscopy (cryo-EM) has greatly contributed to our understanding of the structure–function relationship of the complex Piezo channels [25, 26]. Ge **gpeng et al. [27] reported the structure of mouse Piezo1 in 2015 for the first time. Using protein engineering, negative-staining electron microscopy, cryo-EM, and X-ray crystallography, they found that Piezo1 is a huge channel protein and has a three-bladed, propeller-like homotrimeric architecture with a central ion-conducting pore module topped with an extracellular cap domain structure containing 2547 amino acids. Subsequent studies resolved 26 out of the 38 TMs in each blade and central pore structure of Piezo1 and analyzed the gating mechanism of Piezos from the high-resolution results in conjunction with mutagenesis experiments that altered key amino acids [25, 28,29,30] (Fig. 1).
Structure of 38-TM topology model: a A cartoon model showing a cylindrical helix displayed a subunit with a single THU and major structural domains. b A 38-TM topology model for Piezo ion channels. (adapted from Zhao et al. [30])
Mouse Piezo2, with 2822 residues, shares about 42% of its sequence with Piezo1, and both Piezo1 and Piezo2 have remarkable 38-TM topological organization. The 38-TM topological organization includes the N-terminal region, the C-terminal pore module, and the connecting beam and anchor domains. The N-terminal region was reported to be essential for mechanical force sensing [27, 31]. On the other hand, a study reported that several pathogenic and gain-of-function mutations are clustered in the C-terminal region. Chimeras between mouse Piezo1 and Drosophila Piezo, and Piezo1 and Piezo2 also verified that the intrinsic inactivation kinetics is primarily determined by the C-terminal pore region. Although Piezo1 and Piezo2 have similar structures, they play different roles in the respiratory system.
Piezo1 in the respiratory system
The expression of Piezo1 in the respiratory system
Piezo1 ion channel is a determinant of vascular architecture during early development. It is readily expressed in a variety of ECs, especially in endothelium and endocardium, that are a part of the vascular system and can directly sense physiological shear stress in the vascular system [32]. The pulmonary circulation is a low-pressure vascular bed in which pulmonary ECs are subjected to mechanical forces during lung expansion [33]. Piezo1 is also expressed in pulmonary ECs (Fig. 2, Table 1). It was speculated that conditional knockout of endothelial Piezo1 in mice might result in death at E9.5-E11.5 of embryonic development [34]. Piezo1 is also highly expressed in smooth muscle cells (SMCs) of small-diameter arteries and is involved in arterial remodeling [18, 33] (Fig. 2, Table 1). Besides, smooth-muscle-specific Piezo1 knockout could impair the activity of stretch-activated ion channels [18]. Moreover, Piezo1 is expressed in lung epithelial cells to regulate cell division and crowding [35, 36]. It is possible that depletion of epithelial Piezo1 leads to a shift in an integrin-dependent pattern of anchorage independence and reduced cell migration/invasion [35]. Also, Piezo1 expressed in myeloid cells acts as a sensor of cyclical pressure [19] to resist the invading bacterial infection.
Piezo1 in pulmonary hypertension
As a pulmonary vasodilator, Piezo1 inhibits pulmonary vasoconstriction [10, 37] and might play a key role in PH under hypoxic conditions [38]. The opening of the Piezo1 ion channel in SMCs has a trophic effect on resistant arteries, influencing their diameter and wall thickness in hypertension [18], which might due to the increased the influx of Ca2+ and activation of transglutaminase II. At the same time, Rode et al. [39] claimed that Piezo1 could elevate blood pressure during exercising while does not affect blood pressure during inactive. It is possible that increased blood flow in exercise increases Piezo1-mediated membrane depolarization, which in turn activates voltage-gated Ca2+ channels in adjacent vascular smooth muscle, leading to vasoconstriction.
On the other hand, a study showed that compared to normal participants, patients with intrapulmonary arterial hypertension exhibited significantly elevated Piezo1 levels in pulmonary artery endothelial cells (PAECs) (1.00 ± 0.09 vs 6.20 ± 1.78, n = 5, P < 0.01) [38]. Similarly, Wang et al. [40] demonstrated that Piezo1 was significantly upregulated in PAECs of patients and mice with idiopathic pulmonary hypertension. They reported that the level of Piezo1 in PAEC was higher in hypo-osmotic (200 mOsm/kg) solution. In a hypo-osmotic environment, Piezo1 in ECs could enhance the membrane stretch and Ca2+-dependent phosphorylation of AKT or ERK [41], and subsequently upregulates expression of Notch ligands (Jag-1/2, and DLL4) in PAECs [40]. Audrey Lhomme et al. used mouse and human PAECs to investigate the effect of Piezo1 channel activation on pulmonary vascular tone and found that intrapulmonary vascular relaxation could be promoted via Piezo1 channels by controlling endothelial [Ca2+]i and NO production [42]. However, they found that the magnitude of the depolarization of Yoda1 (Piezo1 ion channel activator) in human PAECs was much smaller than that reported by Rode et al. [39] for mesenteric artery depolarization (5 mV vs 20 mV). This difference may be related to the increased presence of Ca2+-activated potassium channels in PAECs, whose activation masks the depolarizing influx of sodium through Piezo1 channels [43].
Piezo1 in pulmonary edema
Activation of Piezo1 in ECs induced by elevated pulmonary microvascular pressure mediates capillary ‘stress failure’ and pulmonary edema [20, 34]. Pulmonary edema is the result of disruption of the lung endothelial barrier, particularly the disruption of endothelial adhesion junctions (AJs) 44,45. Friedrich et al. [20] demonstrated that Piezo1 is associated with increased pulmonary microvascular pressure and endothelial barrier disruption. They conditionally knocked out Piezo1 in pulmonary ECs (Piezo1iΔEC) and elevated pulmonary microvascular pressure and found that Piezo1iΔEC mice did not develop pulmonary endothelial barrier leakage and edema. Endothelial AJs proteins might exhibit a unique restrictive barrier in Piezo1iΔEC mice. The authors suggested that Piezo1 signals pulmonary vascular hyperpermeability by promoting the internalization and degradation of endothelial AJs (calmodulin). The absence of Piezo1 or inhibition of calpain in ECs prevents the reduction in AJ proteins. Zhong et al. [46] demonstrated that in mechanically ventilated mice, the lung vascular permeability was significantly increased in Piezo1iΔEC mice than in Piezo1fl/fl mice, which could respond to high-volume mechanical ventilation. They showed that the downregulation of endothelial Piezo1 signaling might be an essential factor in the pathogenesis of ventilator-induced lung injury [46]. Influence may arise from the function of calpain in endothelial, which could cleave Src kinase to restore stability of the endothelial barrier [46].
Piezo1 in lung cancer
Lung cancer is one of the most common fatal diseases in the world. Expression of Piezo1 in epithelial cells was also reported to influence the development of non-small cell lung cancer (NSCLC) [35]. Studies have analyzed the mRNA expression of Piezo1 in NSCLC and normal tissues and found that the expression was significantly lower in NSCLC compared to the normal tissues [35, 47]. Database with 1432 NSCLC patients reported that high mRNA expression of Piezo ion channel correlated with better overall survival of NSCLC patients [HR 0.79 (0.64–0.89)], especially in lung adenocarcinoma [HR 0.62 (0.46–0.84)] and female patients [HR 0.68 (0.51–0.89)] [47]. This may be due to the role of Piezo to regulate cell migration and tumor growth [35].
Piezo1 in lung inflammation
Direct recognition of invading pathogens by innate immune cells is a key factor in the inflammatory response. Solis et al. demonstrated that Piezo1 expressed in myeloid cells might be associated with inflammation in the lung [19]. They reported that the knockdown of Piezo1 in myeloid cells showed diminished lung inflammation in the presence of bacterial infection in mice. A possible reason was that Piezo1 in myeloid cells could respond to cyclical hydrostatic pressure (CHP), which triggered the upregulation of proinflammatory genes such as IL1β and Cxcl10 via lipopolysaccharide (LPS). Moreover, mice with conditionally Piezo1-deficient myeloid cells (Piezo1ΔLysM) showed a marked susceptibility to Pseudomonas aeruginosa infection. The authors also found that despite the absence of the classical pattern recognition receptor signaling pathway, mechanical stimulation of macrophages and monocytes triggered an efficient and selective expression of pro-inflammatory and chemoattractant mediators in vitro. This inflammatory mechanosensory response is entirely dependent on Piezo1. It might result from impaired expression of monocyte-dependent endothelin-1, stabilization of HIF1α, and production of proinflammatory mediators.
Moreover, authors used bleomycin-induced pulmonary fibrosis model to investigate if Piezo1 in myeloid cell could drive autoinflammatory disease and found that there was lower level of lung damage in Piezo1ΔLysM mice [19]. Piezo1 signaling in myeloid cells exacerbates pulmonary fibrosis, suggesting that mechanosensation in the altered microenvironment of fibrotic tissue can itself trigger auto-inflammation.
Piezo2 in the respiratory system
The expression of Piezo2 in extrapulmonary and intrapulmonary airways
Piezo2 is an important mechanotransduction channel for a tactile response, proprioception, and mechanical pain in various types of neurons [48,49,50]. Unlike in the case of tactile response and proprioception, Piezo2 activity in sensory neurons that innervate the lung is critical for sensing organ stretch in adult mice [21]. Researchers used a lineage tracing method and detected GFP expression in the jugular-nodose ganglia complex, in which vagal cell bodies are located [21] (Fig. 3, Table 2). Sensory neuron-specific conditional knockout Piezo2 mice exhibit reduced adaptive neurons, mechanosensitive endings, and nerve endings impulse firing in response to mechanical stimuli [51].
A recent study was conducted to verify the distribution of Piezo2 in lung sections from healthy subjects and emphysema and lung fibrosis patients. They found that Piezo2 staining was localized to bronchial epithelial cells, macrophages, and smooth muscle cells [52]. Keiko et al. used the Piezo2GFP reporter line to investigate where Piezo2 is expressed in the airway tract of mice and found that the GFP expression was detected in neuroepithelial body cells (NEBs) [21] (Fig. 3, Table 2).
Role of Piezo2 in establishing respiration
Piezo2 could be critical for establishing newborn respiration. Mice with Piezo2 knockout in the jugular-nodose ganglia, trigeminal nerve, and DRG often die within 24 h after birth due to respiratory failure and inability to suckle [21, 53]. In humans, infants born with Piezo2 deficiency require emergency oxygen support at birth and continue to exhibit diminished respiratory support and shallow breathing throughout life [7, 54]. Delle et al. reported 10 patients with double allele deletion of Piezo2 who developed muscle atrophy with perinatal respiratory distress; however, all patients recovered respiratory function on their own within 24 h after birth [55]. These results suggested that Piezo2 is required for the transition from umbilical cord oxygen supply to respiration in neonatal mammals. Proper lung expansion and initiation of effective respiration in neonatal mice depended on the expression of Piezo2 in neural crest origin [21]. However, the stimuli that drive the firing of these neurons remain unknown, and the role of mechanical transduction in neonatal respiratory processes is currently unclear.
Piezo2 channel activation by mechanical stimulation
During respiration, the lungs experience extensive mechanical forces, and these mechanical forces in the airway are thought to be cues that trigger physiological responses in the lungs [22]. The airway is innervated by both the vagus nerve and spinal sensory nerves [56, 57]. Vagal sensory neurons transmit basic sensory information (e.g., lung inflation [22, 56], arterial oxygen pressure, etc.) to the respiratory center in the brainstem. Studies showed that compared to rats with an intact vagal nerve, vagotomized rats had a 1.7-fold increase in tidal volume and a 2.4-fold decrease in the respiratory rate in the absence of appropriate vagal sensory feedback [56, 58]. Similarly, the conditional knockout of Piezo2 in vagal sensory neurons led to a 1.3-fold increase in the tidal volume. A recent study identified two distinct subtypes of vagal sensory neurons that innervate the lung and have unique functions: activation of neuron P2ry1, which expresses purinergic receptors and induces apnea, and the activation of neuron Npy2r, which expresses neuropeptide receptors and leads to rapid, shallow breathing [59].
Overactivation of Piezo2+ vagal neurons might overtly increase lung stretch responses in patients and lead to respiratory complications. A study reported that ablation of Piezo2 in sensory neurons of adult mice resulted in decreased neuronal response to lung inflation and impaired Hering-Breuer reflex [21]. They measured Hering-Breuer mechanoreflex in the conditional knockout of Piezo2 in nodose-ganglia complex and found that Piezo2-deficient mice had normal respiration as their lungs were inflated [21]. This indicated that Piezo2 expressed in the nodose-ganglia complex might be the major mechanotransducer required for the Hering-Breuer reflex. Moreover, abnormal activation of Piezo2 might be related to the occurrence of chronic pulmonary obstructive disease (COPD), in which the Hering-Breuer reflex is impaired [60].
Piezo2 channel activation by chemical stimulation
Piezo2 expressed in NEBs might affect respiration. In the lungs, NEBs consist of clustered pulmonary neuroendocrine cells (PNECs), which represent a rare and evolutionarily conserved airway epithelial cell population [61]. The close association of Piezo2 with specific excitable NEBs is highly suggestive of a more complex signaling pathway. NEBs are organized in close association with a large number of nerve endings and are suitable to perceive changes in the airway environment and transmit this information to the central nervous system [61, 62]. They could be activated by a variety of stimuli and are thought to monitor all aspects of lung physiology, including inhalation, chemical, and mechanical changes [63]. According to the "chemical" hypothesis of mechanotransduction, NEBs should be able to respond to mechanical stimuli by secreting chemical signals and activating their respective molecular receptors on myelinated vagal sensory terminals [64, 65]. They could release bioactive substances, such as serotonin (5-hydroxytryptamine, 5-HT), substance P, and calcitonin gene-related peptide upon stimulation, and selectively engage mainly with vagal afferent nerve endings.
Increased NEBs and even increased PNEC products have been recognized in several respiratory diseases previously, such as bronchopulmonary dysplasia, COPD, ARDS [66], and allergic asthma [67]. There was a rare study that discussed the involvement of Piezo2, expressed in NEBs, in these diseases. On the other hand, the role of Piezo2 in NEBs might be similar to that in enterochromaffin cells [68]. Alcaino et al. showed that in visceral sensory neurons, Piezo2 is expressed in a subset of intestinal epithelial enteroendocrine cells and intestinal chromophores [17]. This subset of cells could generate a rapid inward ionic current that increases calcium ions in Piezo2-dependent intestinal epithelial enteroendocrine cells, while leading to a Piezo2-dependent mechanical sensitive 5-HT release, and regulating intestinal fluid secretion.
Respiratory role of Piezo2 expressed in brain tissues
Breathing does not only help transport oxygen to the lungs to keep us alive, it also affects how we think and feel. During breathing, significant changes are observed in the activity of the hippocampus and amygdala in the brain. Christina Zelano et al. reported that when someone inhales, neurons in the olfactory cortex, amygdala, and hippocampus, all of which travel through the brain's limbic system, are stimulated [69]. They claimed that the memory of people might be better during inhalation than exhalation. Obstructive sleep apnea/ hypopnea syndrome (OSAHS) leads to a decrease in intracranial blood oxygen concentration and damages hippocampal neurons. Piezo2 expressed in the hippocampus might explain the relationship between the brain and the respiratory system [70]. However, existing evidence does not prove that OSAHS is related to the expression of Piezo2 in the hippocampus.
Conclusion and future perspective
In conclusion, Piezo ion channels play a mechanosensitive and mechanotransductive role in lung diseases. Alveolar epithelial and endothelial cells experience mechanical forces during respiration. Deficiency of Piezo1 or mutation in Piezo2 leads to impaired epithelial cell adhesion and increased cell migration [35], which may promote tissue repair in ARDS and NSCLC. Although this issue has not been investigated, the alveolar epithelial expression of Piezo1 and Piezo2 may regulate the response to distraction-induced or ventilation-induced lung injury. Therefore, Piezo ion channels may play an important role in multiple aspects of lung biology.
Although our understanding of the mechanisms of mechanotransduction has advanced since the discovery of Piezo channels, several fundamental questions regarding the regulatory role of Piezo channels in the lung remain unanswered. For example, research verifying the mechanism underlying the effect of Piezo2 on the Hering-Breuer reflex is lacking. Similarly, research on Piezos in the pulmonary epithelium and endothelium will yield important insights into lung physiology and pathophysiology.
Availability of data and materials
Not applicable.
Abbreviations
- 5-HT:
-
5-Hydroxytryptamine
- AJ:
-
Adhesion junction
- CHP:
-
Cyclical hydrostatic pressure
- cryo-EM:
-
Cryo-electron microscopy
- COPD:
-
Chronic pulmonary obstructive disease
- DRG:
-
Dorsal root ganglia
- ECs:
-
Endothelial cells
- LPS:
-
Lipopolysaccharide
- MA:
-
Mechanically activated
- NSCLC:
-
Non-small cell lung cancer
- OSAHS:
-
Obstructive sleep apnea/hypopnea syndrome
- PAECs:
-
Pulmonary artery endothelial cells
- PH:
-
Pulmonary hypertension
- PNECs:
-
Pulmonary neuroendocrine cells
- TMs:
-
Transmembrane domains
References
Kefauver JM, Ward AB, Patapoutian A. Discoveries in structure and physiology of mechanically activated ion channels. Nature. 2020;587(7835):567–76.
Ranade Sanjeev S, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87(6):1162–79.
Radin I, Richardson RA, Haswell ES. Moss PIEZO homologs have a conserved structure, are ubiquitously expressed, and do not affect general vacuole function. Plant Signal Behav. 2021;2015:893.
Wang P, Jia Y, Liu T, Jan YN, Zhang W. Visceral mechano-sensing neurons control drosophila feeding by using piezo as a sensor. Neuron. 2020;108(4):640–50.
Murthy SE, Dubin AE, Patapoutian A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol. 2017;18(12):771–83.
Cox CD, Bavi N. Martinac BJArop. Bacterial mechanosensors. 2018;80:71–93.
Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, et al. The Role of PIEZO2 in Human Mechanosensation. N Engl J Med. 2016;375(14):1355–64.
Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci. 2015;18(12):1756–62.
Wu Z, Grillet N, Zhao B, Cunningham C, Harkins-Perry S, Coste B, et al. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nat Neurosci. 2017;20(1):24–33.
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science (New York, NY). 2010;330(6000):55–60.
Douguet D, Honoré E. Mammalian mechanoelectrical transduction: structure and function of force-gated ion channels. Cell. 2019;179(2):340–54.
Lacroix JJ, Botello-Smith WM, Luo Y. Probing the gating mechanism of the mechanosensitive channel Piezo1 with the small molecule Yoda1. Nat Commun. 2018;9(1):2029.
Wu J, Lewis AH, Grandl J. Touch, Tension, and Transduction - The Function and Regulation of Piezo Ion Channels. Trends Biochem Sci. 2017;42(1):57–71.
Florez-Paz D, Bali KK, Kuner R, Gomis A. A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons. Sci Rep. 2016;6:25–31.
Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell. 2014;157(3):664–75.
Marshall KL, Saade D, Ghitani N, Coombs AM, Szczot M, Keller J, et al. PIEZO2 in sensory neurons and urothelial cells coordinates urination. Nature. 2020;588(7837):290–5.
Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci USA. 2018;115(32):E7632–41.
Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, et al. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 2015;13(6):1161–71.
Solis AG, Bielecki P, Steach HR, Sharma L, Harman CCD, Yun S, et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;573(7772):69–74.
Friedrich EE, Hong Z, **ong S, Zhong M, Di A, Rehman J, et al. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc Natl Acad Sci USA. 2019;116(26):12980–5.
Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, et al. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature. 2017;541(7636):176–81.
Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol. 2014;4(1):287–324.
Brown GR, Hem V, Katz KS, Ovetsky M, Wallin C, Ermolaeva O, et al. Gene: a gene-centered information resource at NCBI. Nucleic Acids Res. 2015;43:36–42.
Zhang M, Wang Y, Geng J, Zhou S, **ao B. Mechanically activated piezo channels mediate touch and suppress acute mechanical pain response in mice. Cell Rep. 2019;26(6):1419–31.
Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554(7693):481–6.
Wang L, Zhou H, Zhang M, Liu W, Deng T, Zhao Q, et al. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature. 2019;573(7773):225–9.
Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527(7576):64–9.
Jiang W, De Rosario JS, Botello-Smith W, Zhao S, Lin Y-C, Zhang H, et al. Crowding-induced opening of the mechanosensitive Piezo1 channel in silico. Commun Biol. 2021;4(1):84.
Jiang Y, Yang X, Jiang J, **ao B. Structural designs and mechanogating mechanisms of the mechanosensitive Piezo channels. Trends Biochem Sci. 2021;46(6):472–88.
Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554(7693):487–92.
Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, et al. Ion permeation and mechanotransduction mechanisms of mechanosensitive piezo channels. Neuron. 2016;89(6):1248–63.
Douguet D, Patel A, Xu A, Vanhoutte PM, Honoré E. Piezo ion channels in cardiovascular mechanobiology. Trends Pharmacol Sci. 2019;40(12):956–70.
Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA. 2014;111(28):10347–52.
Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515(7526):279–82.
McHugh BJ, Murdoch A, Haslett C, Sethi T. Loss of the integrin-activating transmembrane protein Fam38A (Piezo1) promotes a switch to a reduced integrin-dependent mode of cell migration. PLoS ONE. 2012;7(7): e40346.
Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, Davey CF, et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543(7643):118–21.
Gottlieb PA, Bae C, Sachs F. Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels (Austin). 2012;6(4):282–9.
Wang Z, Babicheva A, Wu L, Yuan JXJ, Wang JJ. Endothelial Upregulation of Mechanosensitive Channel Piezo1 in Pulmonary Hypertension. Channel. 2019;33(1):82712.
Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ, et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun. 2017;8(1):350.
Wang Z, Chen J, Babicheva A, Jain PP, Rodriguez M, Ayon RJ, et al. Endothelial Upregulation of Mechanosensitive Channel Piezo1 in Pulmonary Hypertension. Am J Physiol Cell Physiol. 2021;321:1010.
Dela Paz NG, Frangos JA. Yoda1-induced phosphorylation of Akt and ERK1/2 does not require Piezo1 activation. Biochem Biophys Res Commun. 2018;497(1):220–5.
Lhomme A, Gilbert G, Pele T, Deweirdt J, Henrion D, Baudrimont I, et al. Stretch-activated Piezo1 channel in endothelial cells relaxes mouse intrapulmonary arteries. Am J Respir Cell Mol Biol. 2019;60(6):650–8.
Vang A, Mazer J, Casserly B, Choudhary G. Activation of endothelial BKCa channels causes pulmonary vasodilation. Vascul Pharmacol. 2010;53(3–4):122–9.
Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009;19(1):8–15.
Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367.
Zhong M, Wu W, Kang H, Hong Z, **ong S, Gao X, et al. Alveolar stretch activation of endothelial piezo1 protects adherens junctions and lung vascular barrier. Am J Respir Cell Mol Biol. 2020;62(2):168–77.
Huang Z, Sun Z, Zhang X, Niu K, Wang Y, Zheng J, et al. Loss of stretch-activated channels, PIEZOs, accelerates non-small cell lung cancer progression and cell migration. Biosci Rep. 2019;39:3.
Szczot M, Liljencrantz J, Ghitani N, Barik A, Lam R, Thompson JH, et al. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci Transl Med. 2018;10:462.
Nickolls AR, Lee MM, Espinoza DF, Szczot M, Lam RM, Wang Q, et al. Transcriptional programming of human mechanosensory neuron subtypes from pluripotent stem cells. Cell Rep. 2020;30(3):932–46.
Romero LO, Caires R, Nickolls AR, Chesler AT, Cordero-Morales JF, Vásquez V. A dietary fatty acid counteracts neuronal mechanical sensitization. Nat Commun. 2020;11(1):2997.
Fernández-Trillo J, Florez-Paz D, Íñigo-Portugués A, González-González O, Del Campo AG, González A, et al. Piezo2 mediates low-threshold mechanically evoked pain in the cornea. J Neurosci. 2020;40(47):8976–93.
Hambright P, Eden K, Rau K, Chappell J, Binks A, LeClair R. Determining the Presence and Expression of Piezo2 in Human Lung Tissue Across Various Pathologies. C57 extracellular matrix and subcellular dynamics in complex lung disease: American Thoracic Society; 2020: 5538–42
Dubin AE, Schmidt M, Mathur J, Petrus MJ, **ao B, Coste B, et al. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep. 2012;2(3):511–7.
Szczot M, Nickolls AR, Lam RM, Chesler AT. The form and function of PIEZO2. Annu Rev Biochem. 2021;90:507–34.
Delle Vedove A, Storbeck M, Heller R, Hölker I, Hebbar M, Shukla A, et al. Biallelic loss of proprioception-related PIEZO2 causes muscular atrophy with perinatal respiratory distress, arthrogryposis, and scoliosis. Am J Hum Genet. 2016;99(5):1206–16.
Kaczyńska K, Szereda-Przestaszewska M. Nodose ganglia-modulatory effects on respiration. Physiol Res. 2013;62(3):227–35.
Belvisi MG. Overview of the innervation of the lung. Curr Opin Pharmacol. 2002;2(3):211–5.
Alexandrova NP, Donina ZA, Danilova GA. Effect of central hypervolemia on respiratory function. J Physiol Pharmacol. 2007;58(1):9–15.
Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal sensory neuron subtypes that differentially control breathing. Cell. 2015;161(3):622–33.
Tryfon S, Kontakiotis T, Mavrofridis E, Patakas D. Hering-Breuer reflex in normal adults and in patients with chronic obstructive pulmonary disease and interstitial fibrosis. Respir Int Rev Thor Dis. 2001;68(2):140–4.
Xu J, Yu H, Sun X. Less is more: rare pulmonary neuroendocrine cells function as critical sensors in lung. Dev Cell. 2020;55(2):123–32.
Brouns I, Oztay F, Pintelon I, De Proost I, Lembrechts R, Timmermans JP, et al. Neurochemical pattern of the complex innervation of neuroepithelial bodies in mouse lungs. Histochem Cell Biol. 2009;131(1):55–74.
Cutz E, Pan J, Yeger H, Domnik NJ, Fisher JT. Recent advances and contraversies on the role of pulmonary neuroepithelial bodies as airway sensors. Semin Cell Dev Biol. 2013;24(1):40–50.
Lembrechts R, Brouns I, Schnorbusch K, Pintelon I, Timmermans JP, Adriaensen D. Neuroepithelial bodies as mechanotransducers in the intrapulmonary airway epithelium: involvement of TRPC5. Am J Respir Cell Mol Biol. 2012;47(3):315–23.
Lembrechts R, Brouns I, Schnorbusch K, Pintelon I, Kemp PJ, Timmermans JP, et al. Functional expression of the multimodal extracellular calcium-sensing receptor in pulmonary neuroendocrine cells. J Cell Sci. 2013;126(Pt 19):4490–501.
Xu J, Xu L, Sui P, Chen J, Moya EA, Hume P, et al. Excess neuropeptides in lung signal through endothelial cells to impair gas exchange. Dev Cell. 2022;57:839.
Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018;360:6393.
Treichel AJ, Finholm I, Knutson KR, Alcaino C, Whiteman ST, Brown MR, et al. Specialized mechanosensory epithelial cells in mouse gut intrinsic tactile sensitivity. Gastroenterology. 2021;162:353.
Zelano C, Jiang H, Zhou G, Arora N, Schuele S, Rosenow J, et al. Nasal respiration entrains human limbic oscillations and modulates cognitive function. J Neurosci. 2016;36(49):12448–67.
Wang J, Hamill OP. Piezo2-peripheral baroreceptor channel expressed in select neurons of the mouse brain: a putative mechanism for synchronizing neural networks by transducing intracranial pressure pulses. J Integr Neurosci. 2021;20(4):825–37.
Acknowledgements
Not applicable.
Funding
This study was funded by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18006).
Author information
Authors and Affiliations
Contributions
XHY drafted the manuscript, YJ and MAJ made substantial contributions to the structure and literature review, GJ gave suggestion for revising the manuscript, WB and KY revised the review and approved the final version. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Expression of Piezo ion channels in human tissues.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
**ong, H., Yang, J., Guo, J. et al. Mechanosensitive Piezo channels mediate the physiological and pathophysiological changes in the respiratory system. Respir Res 23, 196 (2022). https://doi.org/10.1186/s12931-022-02122-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02122-6