Abstract
Diagnosing and treating asthma in paediatric patients remains challenging, with many children and adolescents remaining uncontrolled despite treatment. Selecting the most appropriate pharmacological treatment to add onto inhaled corticosteroids (ICS) in children and adolescents with asthma who remain symptomatic despite ICS can be difficult. This literature review compares the efficacy and safety of long-acting β2-agonists (LABAs), leukotriene receptor antagonists (LTRAs) and long-acting muscarinic antagonists (LAMAs) as add-on treatment to ICS in children and adolescents aged 4–17 years.
A literature search identified a total of 29 studies that met the inclusion criteria, including 21 randomised controlled trials (RCTs) of LABAs versus placebo, two RCTs of LAMAs (tiotropium) versus placebo, and four RCTs of LTRA (montelukast), all as add-on to ICS. In these studies, tiotropium and LABAs provided greater improvements in lung function than LTRAs, when compared with placebo as add-on to ICS. Although exacerbation data were difficult to interpret, tiotropium reduced the risk of exacerbations requiring oral corticosteroids when added to ICS, with or without additional controllers. LABAs and LTRAs had a comparable risk of asthma exacerbations with placebo when added to ICS. When adverse events (AEs) or serious AEs were analysed, LABAs, montelukast and tiotropium had a comparable safety profile with placebo.
In conclusion, this literature review provides an up-to-date overview of the efficacy and safety of LABAs, LTRAs and LAMAs as add-on to ICS in children and adolescents with asthma. Overall, tiotropium and LABAs have similar efficacy, and provide greater improvements in lung function than montelukast as add-on to ICS. All three controller options have comparable safety profiles.
Similar content being viewed by others
Lay summary
It can be difficult for doctors to decide which treatment is best to prescribe to children and adolescents with asthma to help reduce their symptoms. In this review, we weigh up the available evidence on three asthma treatments that work in different ways. We looked at two types of inhalers and one type of medicine that is either swallowed as a tablet or granules. The two inhalers helped to improve lung function more than the oral medication, which may be due to their different modes of action. All three treatments were found to be as safe as a placebo.
Introduction
Asthma is one of the most prevalent chronic diseases in childhood [1], yet diagnosing and treating asthma in children remains challenging. Poor control of asthma in children and adolescents is common and represents a considerable cause of morbidity [2, 3]. In addition to its physical effects, the disease can have an emotional impact on the patient and cause a great burden for patients’ families and the community [1]. There is, therefore, a need for more pharmacological options to improve asthma control in children and adolescents whose symptoms are not fully treated with inhaled corticosteroids (ICS).
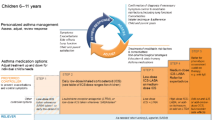
Selecting the most appropriate add-on treatment to manage and reduce asthma symptoms in children and adolescents whose asthma remains uncontrolled despite treatment can be challenging. The Global Initiative for Asthma (GINA) recommends that patients with asthma who continue to experience symptoms and/or exacerbations on low-dose ICS have their ICS dose increased and combined with long-acting β2-agonists (LABAs) or other controllers in a step-wise fashion (Fig. 1). Further controller medications include long-acting muscarinic antagonists (LAMAs; e.g. tiotropium), leukotriene receptor antagonists (LTRAs), theophylline and biologics [4]. GINA also recommends as-needed low-dose ICS/formoterol as reliever therapy in all patients > 12 years of age, with short-acting β2-agonists (SABAs) recommended as an alternative reliever medication [4], although it should be noted that the recommendation for children is to ensure additional ICS is taken whenever the SABA reliever is given [4]. The goals of asthma management are aligned across all age groups: namely, to achieve good symptom control, maintain normal activity levels, lung function and development, and minimise future risk of exacerbations and side effects associated with medication [4].
GINA treatment recommendations for patients aged ≥ 5 years, 6–11 years and ≥ 12 years [4]. FEV1, forced expiratory volume in 1 s; GINA, Global Initiative for Asthma; HDM, house dust mite; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroids; SABA, short-acting β2-agonist; SLIT, sublingual immunotherapy
Previous studies have demonstrated the efficacy and safety of LABAs as add-on to ICS compared with placebo [5, 6]. LABAs are available both as single therapy to be taken as add-on to ICS, or as dual therapy, where ICS and LABA are delivered in the same device. Single-therapy LABAs are indicated as add-on treatment to ICS for patients aged from 4 years in Europe and the USA [7,8,9,10].
Tiotropium, an alternative add-on treatment to ICS, is a LAMA that is efficacious in clinical trials in adolescents and children with asthma as add-on to ICS [11, 12] or to ICS with other controllers [13, 14]. In the European Union, it is now indicated as add-on maintenance treatment in patients aged 6 years and older with severe asthma who experienced one or more severe asthma exacerbations in the past year [15]. In the USA, tiotropium is indicated in the long-term, once-daily maintenance treatment of asthma in patients aged 6 years and older [16].
The LTRA montelukast is indicated in the treatment of asthma as an add-on therapy in paediatric patients with mild-to-moderate persistent asthma who are inadequately controlled on ICS and in whom SABAs provide inadequate control [17]. It can also be tried as an alternative to ICS in patients with mild-to-persistent asthma who do not have a history of asthma attacks and have trouble using inhaled medications, and is indicated for the prophylaxis of asthma in patients aged at least 2 years [18]. Montelukast oral granules are indicated in patients aged between 6 months and 5 years [19].
Despite the availability of these controller medications, few studies have directly compared their efficacy in adolescents and children with asthma. A number of systematic reviews have compared the effects of LAMAs, LABAs and LTRAs as add-on to ICS in patients with asthma [6, 20,21,22], although reviews in children aged < 12 years or adolescents aged 12–18 years are limited. Moreover, none have been published that compare the efficacy and safety of all three add-on treatments within one review in patients aged ≤18 years. More systematic reviews and treatment recommendations have been published for patients aged ≥12 years than those for younger patients. As such, there is a need for an up-to-date review of the literature related to the treatments available as add-on to ICS in paediatric patients with asthma.
The aim of this literature review is to compare the efficacy and safety of three controller options (LAMA, LABA and LTRA) as add-on to ICS in adolescents and children aged 4–17 years with asthma. We compare the magnitude of forced expiratory volume in 1 s (FEV1) improvements with each drug class, their effects on exacerbations, and the proportion of patients with adverse events (AEs) and serious AEs (SAEs).
Methods
We carried out an electronic literature search of the Cochrane Database of Systematic Reviews in December 2018 to identify any previously published systematic reviews, which were then manually checked for relevance. We then searched PubMed for articles published since the search date detailed within the systematic review.
The inclusion criteria for this review were randomised controlled trials (RCTs) of at least 4 weeks in duration in children and adolescents aged 4–17 years. The types of intervention included LABA, LAMA or LTRA versus placebo, or versus each other, added onto ICS, compared with the same dose of ICS alone. The primary outcome of interest was lung function, measured using FEV1. For FEV1, we included percent predicted as well as absolute values, as this has the advantage of removing physical confounding factors, particularly when comparing studies with different age groups of children. Secondary outcomes included exacerbations requiring oral corticosteroids (OCS), and proportion of patients reporting AEs and SAEs.
Data were extracted from published articles in PubMed and publicly available data online. We also checked the reference lists of the systematic reviews for any additional data for endpoints that were not described in the systematic reviews. Results were compared with data from tiotropium trials in paediatric patients (PensieTinA- [NCT01277523], VivaTinA- [NCT01634152], RubaTinA- [NCT01257230] and CanoTinA-asthma® [NCT01634139]).
We used the following search strings:
Studies of LABA as add-on to ICS
((((((((((clinical trial[MeSH Terms]) OR clinical trial) OR clinical study)))))
AND asthma[MeSH Terms]))
AND ((((((((Asthma Control Questionnaire) OR ACQ)) OR ((forced expiratory volume) OR FEV)) OR ((exacerbation) OR worsening)) OR adverse event)))))
AND ((((((((((((((((((child*) OR paediat*) OR pediat*) OR adolesc*) OR infan*) OR young*) OR preschool*) OR “pre school*”) OR pre-school*))))
AND (((((((((seretide) OR symbicort) OR advair) OR viani) OR flutiform))
OR (((((((((((glucocorticoids[MeSH Terms]) OR inhaled corticosteroid*) OR budesonide) OR beclomethasone) OR beclometasone) OR fluticasone) OR triamcinolone) OR flunisolide) OR ciclesonide))
AND (((((((((adrenergic beta 2 receptor antagonists[MeSH Terms]) OR ((((beta*) AND agonist*)) AND ((long-acting) OR “long acting”))) OR ((((beta*) AND adrenergic*)) AND ((long-acting) OR “long acting”))) OR ((bronchodilat*) AND ((long-acting) OR “long acting”))) OR salmeterol) OR serevent) OR *formoterol) OR foradil) OR vilanterol))))))
AND (“2015/02/01”[Date - Publication]: “2018/12/19”[Date - Publication])
Studies of LTRA as add-on to ICS
((((((((((clinical trial[MeSH Terms]) OR clinical trial) OR clinical study)))))
AND asthma[MeSH Terms]))
AND ((((((((forced expiratory volume) OR FEV)) OR ((exacerbation) OR worsening)) OR adverse event)))))
AND ((((((((((((((((((child*) OR paediat*) OR pediat*) OR adolesc*) OR infan*) OR young*) OR preschool*) OR “pre school*”) OR pre-school*))))
AND (((((((((((((glucocorticoids[MeSH Terms]) OR inhaled corticosteroid*) OR budesonide) OR beclomethasone) OR beclometasone) OR fluticasone) OR triamcinolone) OR flunisolide) OR ciclesonide))) AND ((((((((((((leukotriene antagonists[MeSH Terms]) OR LTRA) OR leukotriene*) OR leucotriene*) OR anti-leukotriene*) OR anti-leucotriene*) OR montelukast) OR singulair) OR zafirlukast) OR accolate) OR pranlukast) OR azlaire))))
AND (“2014/07/01”[Date - Publication]: “2018/12/19”[Date - Publication])
Studies of LAMA as add-on to ICS
(((((((((clinical trial[MeSH Terms]) OR clinical trial) OR clinical study)))))
AND asthma[MeSH Terms]))
AND ((((((((Asthma Control Questionnaire) OR ACQ)) OR ((forced expiratory volume) OR FEV)) OR ((exacerbation) OR worsening)) OR adverse event)))))
AND ((((((((((((((((((child*) OR paediat*) OR pediat*) OR adolesc*) OR infan*) OR young*) OR preschool*) OR “pre school*”) OR pre-school*))))
AND (((((((((((((glucocorticoids[MeSH Terms]) OR inhaled corticosteroid*) OR budesonide) OR beclomethasone) OR beclometasone) OR fluticasone) OR triamcinolone) OR flunisolide) OR ciclesonide))) AND (((((((((((((((((((((((muscarinic) AND antagonist*)))) AND (((long-acting) OR “long acting”)))))) OR ((antagonists, muscarinic[MeSH Terms]) AND (((long-acting) OR “long acting”)))))) OR LAMA) OR glycopyrronium) OR aclidinium) OR tiotropium) OR umeclidinium) OR NVA237) OR seebri) OR LAS34273) OR turdorza) OR pressair) OR eklira) OR genuair) OR spiriva) OR GSK573719)))
The literature searches were reviewed from the title, abstract or descriptors, and all studies that were not RCTs or that clearly did not fit the inclusion criteria were excluded. Data were analysed from the articles deemed appropriate for inclusion. Where appropriate, we performed a meta-analysis using the Cochrane statistical package RevMan 5, assuming equivalence if the risk ratio estimate and its confidence interval (CI) were between 0.9 and 1.1. The risk of bias was assessed using a domain-based evaluation, in line with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions [23]. Various domains, including allocation concealment and blinding, were judged as being low, unclear or high. Studies were deemed to be of high methodological quality when the reported randomisation and blinding procedures were adequate and at a low risk of bias, with balanced group attrition.
Results
Identification of relevant articles
A literature search identified four systematic reviews (Fig. 2). Of these, one compared RCTs of LABAs as add-on to ICS, published up to February 2015, and was included in the review [24]. Three of the systematic reviews compared LTRAs with placebo as add-on to ICS. Of these, two were included in this review [25, 26], with the most recent studies published up to July 2014. One systematic review comparing LTRAs with placebo [27] was excluded as data from the included studies were already covered in the 2010 systematic reviews. No systematic reviews were identified that compared LAMAs with placebo, or LABAs, LTRAs or LAMAs directly with one another. We reviewed the three systematic reviews and analysed the relevant studies for inclusion in this review.
Additional literature searches identified 73 articles, published since February 2015, comparing LABAs with placebo, of which two met the inclusion criteria for this review [28, 29]. Twenty-three articles published since July 2014 were identified comparing LTRA with placebo, of which one met the inclusion criteria for this review [30]. An additional 16 articles comparing LAMAs with placebo were identified, of which two met the inclusion criteria for this review [11, 31]. We also included two studies in which patients received tiotropium as add-on to ICS plus other controllers, which were not identified in the literature search as the search strings excluded additional controller medications to LAMA [13, 14]. There were no additional studies identified that compared LABAs, LTRAs or LAMAs directly with one another. In total, 29 studies were included in this review.
The designs of all included studies are summarised in Table 1. All studies were randomised, and most were double-blinded and parallel-group in design, ranging from 4 to 54 weeks in duration. Participants were 4–18 years of age. Primary outcomes included safety and lung function.
An overview of judgements on domains related to risk of bias is reported in Table 2. Most bias items were deemed to be of low or unclear risk.
FEV1 results
The LABA studies included in the Cochrane meta-analysis present a combination of peak and trough FEV1 measurements, and some articles do not specify at what time point the measurement was taken [24]. For this reason, we present both peak and trough FEV1 response data where available.
FEV1: absolute difference in litres
We performed a meta-analysis of nine LABA studies. There was a treatment difference in FEV1 of 0.07 L (95% CI 0.05, 0.08) (Fig. 3). Excluding the two outliers (a vilanterol study that found no improvement [− 0.06 to 0.02 L] [28] and a very small [n = 21] salmeterol study [0.42 L (95% CI 0.21, 0.63)] [46]), mean treatment differences were 0.04–0.13 L (Fig. 3). None of the included LTRA studies presented data for change from baseline in litres.
For the LAMA studies, we pooled the data for studies where tiotropium was the only add-on therapy (no additional LABA add-on therapy permitted) (RubaTinA-asthma® and CanoTinA-asthma®) [11, 31] and presented both peak and trough results for tiotropium Respimat® 5 μg and 2.5 μg (Fig. 4). Peak FEV1 was defined as the maximum FEV1 within 3 h after dosing and trough FEV1 was defined as the pre-dose FEV1 measured 24 h after the previous drug administration and 10 min prior to the evening dose of the patient’s usual asthma medication. We did the same for studies where tiotropium Respimat® was the third or even fourth controller (PensieTinA-asthma® and VivaTinA-asthma®) (Fig. 4). None of the included studies investigated tiotropium delivered via the HandiHaler® device [13, 14].
Pooled treatment difference in peak (a) and trough (b) FEV1 response between tiotropium Respimat® and placebo added to ICS for patients with symptomatic moderate asthma and patients with symptomatic severe asthma. CI, confidence interval; FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroid
FEV1 improvements versus placebo with tiotropium Respimat® as add-on to ICS in studies of children and adolescents with symptomatic moderate asthma were 0.159–0.168 L for peak FEV1 and 0.105–0.118 L for trough FEV1 (Fig. 4). For studies in children and adolescents with symptomatic severe asthma, FEV1 improvements versus placebo were 0.074–0.117 L for peak FEV1 and 0.064–0.071 L for trough FEV1 (Fig. 4).
FEV1 response: percent predicted
The Cochrane analysis of LABA studies (Table 3) found an improvement in FEV1 percent predicted with LABAs added to ICS versus ICS of 2.99% (95% CI 0.86, 5.11; n = 534) [24]. Results from individual LABA studies are also detailed in Table 3. Improvements in peak FEV1 percent predicted with tiotropium added to ICS versus ICS were 4.07–7.70%, and 2.85–5.05% for trough FEV1; improvements with tiotropium added to ICS with other controllers were 1.64–6.33% for peak FEV1 and 0.83–3.85% for trough FEV1.
The treatment difference with montelukast added to ICS compared with ICS alone varied, with the systematic review finding an improvement of 0.09% (95% CI − 0.07 to 0.25; n = 188) [25] and individual studies mostly ranging from 1.3 to 2.6%. One single-centre study found an improvement of 10.8% with montelukast compared with ICS, but this was a small, 4-week study (n = 24), and no confidence intervals or statistical comparison was available [50].
Exacerbations requiring OCS
The Cochrane analysis of LABA studies (n = 1669) found no difference in the risk of exacerbations requiring OCS between LABAs plus ICS compared with ICS alone (risk ratio 0.95; 95% CI 0.70, 1.28) (Table 4) [24]. The individual studies were quite variable, with study durations of 4–54 weeks. We found no additional studies reporting on exacerbations requiring OCS in our literature search.
Risk ratios were not available for the tiotropium studies, but the proportion of patients with exacerbations requiring OCS was low in all of the studies (Table 4). Tiotropium provided improvements in time to first exacerbation requiring OCS when added onto ICS versus placebo, with hazard ratios of 0.23–1.14, and 0.40–2.06 when added on to other controllers.
The systematic review of the LTRA studies showed no difference between montelukast and placebo on top of ICS, but the authors noted that there was evidence of statistical heterogeneity [25]. The network meta-analysis found no difference between montelukast and placebo (odds ratio 0.94; 95% CI 0.58, 1.45) [26]. One 7-month study found fewer exacerbations with montelukast than with placebo as add-on to ICS (odds ratio 0.26; 95% CI 0.09, 0.76) [30].
Adverse events and serious adverse events
The proportion of patients experiencing AEs or SAEs with the addition of LABA to ICS was broadly similar, with some variations in the proportion of patients with AEs or SAEs between studies (Table 5).
There was no increase in the number of patients with AEs or SAEs with tiotropium compared with placebo as add-on to ICS or add-on to ICS plus other controllers (Table 5).
There were limited data on the number of patients with AEs in the montelukast analyses; the study that did report the proportion of patients with AEs showed no significant difference between montelukast and placebo as add-on to ICS (Table 5). There were insufficient data to make a comment on SAEs in the montelukast trials.
Efficacy and safety of tiotropium Respimat® as add-on to ICS and additional controller medications
In studies where tiotropium Respimat® was added onto ICS and additional controller medications (PensieTinA-asthma® and VivaTinA-asthma®) [13, 14], the effect size for both lung function and exacerbations requiring OCS was comparable with the studies where tiotropium was the only controller [11, 31], or where LABA or LTRA were added onto ICS [24,25,26, 28,29,30]. In addition, the studies demonstrated comparable safety with placebo [13, 14].
Discussion
In this literature review, the addition of once-daily tiotropium (with or without other controllers) and twice-daily LABAs to ICS in children and adolescents provided similar improvements in lung function [11, 13, 14, 24, 28, 29, 31], and greater improvements than with once-daily LABA vilanterol added onto ICS [28]. Data reporting on the effect of LTRAs as add-on to ICS on lung function were somewhat inconsistent, yet a previous systematic review found no improvement with montelukast compared with placebo when added to ICS [25], so it may be appropriate to suggest that twice-daily LABAs and tiotropium are more effective at improving lung function in adolescents and children as add-on to ICS. This assumption could be further clarified if future studies directly compared tiotropium, LABAs and LTRAs as add-on to ICS.
An additional endpoint that we analysed in this review was asthma exacerbations. However, the exacerbation data were more difficult to interpret, as the studies were of different durations and not necessarily powered to show a treatment difference in exacerbation frequency. Powering a study in paediatric patients to assess asthma exacerbations may present ethical considerations, with patients receiving placebo or care that is inconsistent with the best proven method, potentially being exposed to unnecessary risk and harm, especially where exacerbation events are expected [52]. In addition, not all studies included a risk ratio, making the comparison of data difficult. However, in the tiotropium trials, where exacerbations were included as a safety endpoint, it was possible to demonstrate that tiotropium provided a reduction in the risk of exacerbations requiring OCS when added onto ICS, either alone or with additional controller treatments, compared with placebo [11, 13, 14, 31]. Although the results from the individual studies of LABA as add-on to ICS varied, the previously published Cochrane review by Chauhan et al. suggested that LABAs and placebo have a comparable risk of asthma exacerbation [24]. In regards to the effect of LTRAs on asthma exacerbations, the data were more inconclusive. The one RCT included on LTRAs reported that montelukast reduced the risk of exacerbations compared with placebo. However, the sample size was small, with only 76 participants [30]. The two systematic reviews reported no reduction in the risk of exacerbations compared with placebo; however, the width of the CIs suggests a large spread of data [25, 26]. It could therefore be suggested that the highest quality of evidence was for the trials investigating LABA or LAMA as add-on to ICS.
The safety data showed no increase in the proportion of patients reporting AEs or SAEs with LABAs or with tiotropium when added to ICS [11, 13, 14, 24, 28, 29, 31]. The available data for LTRAs were limited, but suggested no increase in the proportion of patients with AEs with montelukast compared with placebo as add-on to ICS [49]. However, it should be noted that previous post-marketing studies have suggested that paediatric patients receiving montelukast are more likely to report neuropsychiatric AEs than those receiving ICS [53, 54]. Therefore, the results from this review indicate that LABAs, LTRAs and LAMAs all have a comparable safety profile to placebo, but other real-world and post-marketing evidence should also be considered.
This literature review aims to provide an up-to-date overview of the efficacy and safety of three classes of drugs that are options for adding onto ICS in adolescents and children with asthma. The strength of the study is that this is the first literature review and meta-analysis to collate and compare the efficacy and safety of LABAs, LTRAs and LAMAs in children and adolescents in one review. Previous reviews have compared the efficacy and safety of LABAs and LAMAs, or LABAs and LTRAs, in adolescents aged over 12 years and in adults, but none has compared all three therapeutic options in one review, and none has done so for this patient population in children and adolescents aged 4–17 years.
We have focused on a limited number of endpoints that are considered important in the treatment of asthma such as lung function, exacerbations and AEs. However, there is considerable variability in the methodology and definition of these endpoints between studies, making the comparison of data more difficult. There were only a limited number of montelukast studies in children that met the inclusion criteria, so LTRA data are lacking for some endpoints. For example, for the LABA studies, we were able to perform a meta-analysis of absolute change in lung function in litres, but LTRA studies only reported lung function change in percent predicted. Moreover, when extracting the FEV1 data from the various studies, the time point of the measurement in relation to drug administration (i.e. peak/trough) was not always clear. Only the LAMA studies reported whether FEV1 was peak (defined as the maximum FEV1 within 3 h after dosing) or trough FEV1 (defined as the pre-dose FEV1 measured 24 h after the previous drug administration and 10 min prior to the evening dose of the patient’s usual asthma medication). As Fig. 4 demonstrates, there are differences between the responses depending on when the measurement is taken, with peak FEV1 (Fig. 4a) values higher than the equivalent trough FEV1 (Fig. 4b) values. Therefore, it is possible that some of the between-study differences in FEV1 response for LABAs and LTRAs may be attributable to the time point at which the measurement was taken, but this cannot be confirmed.
In light of the extension of the tiotropium label and the most recent treatment guidelines for children with asthma [4], the results provide support for the use of tiotropium as add-on therapy in adolescents and children with asthma aged 4–17 years. The results are in agreement with those of a recently published systematic review that compared LABAs with LAMAs in patients aged over 12 years [22]. The authors reported that use of LAMA as add-on to ICS was associated with a lower risk of asthma exacerbations compared with placebo, and had a comparable benefit to LABA on lung function. The authors note that their review was designed and conducted in patients aged 12 years and over because tiotropium was not approved in patients aged less than 12 years at the time the study was undertaken [22]. In addition, it does not review the literature on LTRAs as an add-on treatment.
In conclusion, tiotropium and LABAs have similar efficacy, and provide greater improvements in lung function than montelukast as add-on to ICS in children and adolescents with asthma. All three controller options have comparable safety profiles. The results of our literature review in patients aged 4–17 years provide needed additional information, and further supports the use of tiotropium in children and adolescents with asthma. The clinical decision on the preferred add-on therapy should also take into account patient phenotype and comorbidities, dose regimen and frequency, the availability of combination therapy, and the delivery device, although more research is required in these younger age groups.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- AE:
-
Adverse event
- CI:
-
Confidence interval
- FEV1 :
-
Forced expiratory volume in 1 s
- GINA:
-
Global Initiative for Asthma
- ICS:
-
Inhaled corticosteroid
- LABA:
-
Long-acting β2-agonist
- LAMA:
-
Long-acting muscarinic antagonist
- LTRA:
-
Leukotriene receptor antagonist
- OCS:
-
Oral corticosteroid
- RCT:
-
Randomised controlled trial
- SABA:
-
Short-acting β2-agonist
- SAE:
-
Serious adverse event
References
European Respiratory Society. European Lung white book – Chapter 11 Childhood asthma. https://www.erswhitebook.org/chapters/childhood-asthma/. Accessed 30 Apr 2018.
Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783–800.
Ferrante G, Grutta S. Reasons for inadequate asthma control in children: an important contribution from the “French 6 Cities Study”. Multidiscip Respir Med. 2012;7:23.
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention (2019 report). 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed 23 July 2019.
Rodrigo GJ, Castro-Rodriguez JA. Safety of long-acting beta agonists for the treatment of asthma: clearing the air. Thorax. 2012;67(4):342–9.
Ducharme F, NiChroinin M, Greenstone I, Lasserson T. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev. 2010;5:CD005535.
Electronic Medicines Compendium. Foradil. 2016. https://www.medicines.org.uk/emc/product/1030/smpc. Accessed 2 Nov 2018.
Food and Drug Administration. Foradil Certihaler (formoterol fumarate inhalation powder). 2012. https://www.fda.gov/downloads/Drugs/DrugSafety/ucm088602.pdf. Accessed 2 Nov 2018.
Electronic Medicines Compendium. Serevent Accuhaler. 2018. https://www.medicines.org.uk/emc/product/848/smpc. Accessed 2 Nov 2018.
Food and Drug Administration. Serevent Diskus (salmeterol xinafoate inhalation powder). 1998. https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20692S1,2_Serevent_prntlbl.pdf. Accessed 2 Nov 2018.
Hamelmann E, Bateman ED, Vogelberg C, Szefler SJ, Vandewalker M, Moroni-Zentgraf P, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441–450.e8.
Vogelberg C, Moroni-Zentgraf P, Leonaviciute-Klimantaviciene M, Sigmund R, Hamelmann E, Engel M, et al. A randomised dose-ranging study of tiotropium Respimat® in children with symptomatic asthma despite inhaled corticosteroids. Respir Res. 2015;16:20.
Szefler SJ, Murphy K, Harper T, Boner A, Laki I, Engel M, et al. A phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthma. J Allergy Clin Immunol. 2017;140:1277–87.
Hamelmann E, Bernstein JA, Vandewalker M, Moroni-Zentgraf P, Verri D, Unseld A, et al. A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J. 2017;49:1601100.
Boehringer Ingelheim Limited. Summary of Product Characteristics – Spiriva Respimat 2.5 microgram, inhalation solution. 2018. https://www.medicines.org.uk/emc/product/407/smpc. Accessed 30 Apr 2018.
U.S. Food and Drug Administration. Prescribing information for Spiriva® Respimat® (tiotropium bromide) inhalation spray, for oral inhalation. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021936s007lbl.pdf. Accessed 22 Oct 2018.
Electronic Medicines Compendium. Singulair paediatric 5 mg chewable tablets. 2017. https://www.medicines.org.uk/emc/product/197/smpc. Accessed 2 May 2018.
Electronic Medicines Compendium. Singulair paediatric 4 mg tablets. 2017. https://www.medicines.org.uk/emc/product/6500/smpc. Accessed 1 May 2018.
Electronic Medicines Compendium. Singulair paediatric 4 mg granules. 2017. https://www.medicines.org.uk/emc/product/45/smpc. Accessed 1 May 2018.
Anderson DE, Kew KM, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus the same dose of ICS alone for adults with asthma. Cochrane Database Syst Rev. 2015;24(8):CD011397.
Kew KM, Evans DJ, Allison DE, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus addition of long-acting beta2-agonists (LABA) for adults with asthma. Cochrane Database Syst Rev. 2015;2(6):CD011438.
Sobieraj DM, Baker WL, Nguyen E, Weeda ER, Coleman CI, White CM, et al. Association of inhaled corticosteroids and long-acting muscarinic antagonists with asthma control in patients with uncontrolled, persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319(14):1473–84.
The Cochrane Collaboration’s tool for assessing risk of bias. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 edition. London: The Cochrane Collaboration; 2011.
Chauhan BF, Chartrand C, Ni Chroinin M, Milan SJ, Ducharme FM. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;24(11, CD007949).
Castro-Rodriguez JA, Rodrigo GJ. The role of inhaled corticosteroids and montelukast in children with mild-moderate asthma: results of a systematic review with meta-analysis. Arch Dis Child. 2010;95(5):365–70.
Zhao Y, Han S, Shang J, Zhao X, Pu R, Shi L. Effectiveness of drug treatment strategies to prevent asthma exacerbations and increase symptom-free days in asthmatic children: a network meta-analysis. J Asthma. 2015;52(8):846–57.
Chauhan BF, Ben Salah R, Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids in children with persistent asthma. Cochrane Database Syst Rev. 2013;2(10):CD009585.
Oliver AJ, Covar RA, Goldfrad CH, Klein RM, Pedersen SE, Sorkness CA, et al. Randomised trial of once-daily vilanterol in children with asthma on inhaled corticosteroid therapy. Respir Res. 2016;17:37.
Pearlman DS, Eckerwall G, McLaren J, Lamarca R, Puu M, Gilbert I, et al. Efficacy and safety of budesonide/formoterol pMDI vs budesonide pMDI in asthmatic children (6–<12 years). Ann Allergy Asthma Immunol. 2017;118:489–499.e1.
Stelmach I, Ozarek-Hanc A, Zaczeniuk M, Stelmach W, Smejda K, Majak P, et al. Do children with stable asthma benefit from addition of montelukast to inhaled corticosteroids: randomized, placebo controlled trial. Pulm Pharmacol Ther. 2015;31:42–8.
Vogelberg C, Engel M, Laki I, Bernstein JA, Schmidt O, El Azzi G, et al. Tiotropium add-on therapy improves lung function in children with symptomatic moderate asthma. J Allergy Clin Immunol Pract. 2018;6(6):2160–2162.e9.
Berger WE, Leflein JG, Geller DE, Parasuraman B, Miller CJ, O’Brien CD, et al. The safety and clinical benefit of budesonide/formoterol pressurized metered-dose inhaler versus budesonide alone in children. Allergy Asthma Proc. 2010;31(1):26–39.
Eid NS, Noonan MJ, Chipps B, Parasuraman B, Miller CJ, O’Brien CD. Once- vs twice-daily budesonide/formoterol in 6- to 15-year-old patients with stable asthma. Pediatrics. 2010;126(3):e565–75.
Pohunek P, Kuna P, Jorup C, De Boeck K. Budesonide/formoterol improves lung function compared with budesonide alone in children with asthma. Pediatr Allergy Immunol. 2006;17(6):458–65.
AstraZeneca plc. Efficacy and safety of budesonide/formoterol Turbuhaler® (160/4.5 mg b.i.d. delivered dose) compared to budesonide Turbuhaler® (200 mg b.i.d. metered dose) in steroid-using asthmatic adolescent patients. A double-blind, double-dummy, randomised, parallel group, phase III, multicentre study. (ATTAIN STUDY): CR-SD-039-0714. 2003.
Akpinarli A, Tuncer A, Saraclar Y, Sekerel BE, Kalayci O. Effect of formoterol on clinical parameters and lung functions in patients with bronchial asthma: a randomised controlled trial. Arch Dis Child. 1999;81(1):45–8.
Morice AH, Peterson S, Beckman O, Kukova Z. Efficacy and safety of a new pressurised metered-dose inhaler formulation of budesonide/formoterol in children with asthma: a superiority and therapeutic equivalence study. Pulm Pharmacol Ther. 2008;21(1):152–9.
Malone R, LaForce C, Nimmagadda S, Schoaf L, House K, Ellsworth A, et al. The safety of twice-daily treatment with fluticasone propionate and salmeterol in pediatric patients with persistent asthma. Ann Allergy Asthma Immunol. 2005;95(1):66–71.
Carroll WD, Jones PW, Boit P, Clayton S, Cliff I, Lenney W. Childhood evaluation of salmeterol tolerance--a double-blind randomized controlled trial. Pediatr Allergy Immunol. 2010;21(2 Pt 1):336–44.
Lenney W, McKay AJ, Tudur Smith C, Williamson PR, James M, Price DB. Management of Asthma in school age children on therapy (MASCOT): a randomised, double-blind, placebo-controlled, parallel study of efficacy and safety. Health Technol Assess. 2013;17:1–218.
Teper AM, Zaragoza SM, Lubovich S, Rodriguez VA, Venalago C, Kofman CD. Effect of fluticasone propionate (FP) with or without salmeterol (S) on bronchial reactivity (BR) in children with mild to moderate persistent asthma [abstract]. Am Thor Soc Int Conf. 2005;C47.
Murray JJ, Waitkus-Edwards KR, Yancey SW. Evaluation of fluticasone propionate and fluticasone propionate/salmeterol combination on exercise in pediatric and adolescent patients with asthma. Open Respir Med J. 2011;5:11–8.
Pearlman D, Qaqundah P, Matz J, Yancey SW, Stempel DA, Ortega HG. Fluticasone propionate/salmeterol and exercise-induced asthma in children with persistent asthma. Pediatr Pulmonol. 2009;44(5):429–35.
Simons FE, Gerstner TV, Cheang MS. Tolerance to the bronchoprotective effect of salmeterol in adolescents with exercise-induced asthma using concurrent inhaled glucocorticoid treatment. Pediatrics. 1997;99(5):655–9.
Russell G, Williams DA, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Ann Allergy Asthma Immunol. 1995;75(5):423–8.
Langton Hewer S, Hobbs J, French D, Lenney W. Pilgrim’s progress: the effect of salmeterol in older children with chronic severe asthma. Respir Med. 1995;89(6):435–40.
Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch asthma study group. Am J Respir Crit Care Med. 1998;158(1):213–9.
Meijer GG, Postma DS, Mulder PG, van Aalderen WM. Long-term circadian effects of salmeterol in asthmatic children treated with inhaled corticosteroids. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1887–92.
Simons FE, Villa JR, Lee BW, Teper AM, Lyttle B, Aristizabal G, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr. 2001;138(5):694–8.
Miraglia del Giudice M, Piacentini GL, Capasso M, Capristo C, Maiello N, Boner AL, et al. Formoterol, montelukast, and budesonide in asthmatic children: effect on lung function and exhaled nitric oxide. Respir Med. 2007;101(8):1809–13.
Stelmach I, Grzelewski T, Bobrowska-Korzeniowska M, Stelmach P, Kuna P. A randomized, double-blind trial of the effect of anti-asthma treatment on lung function in children with asthma. Pulm Pharmacol Ther. 2007;20(6):691–700.
Cabana MD, Kunselman SJ, Nyenhuis SM, Wechsler ME. Researching asthma across the ages: insights from the National Heart, Lung, and Blood Institute’s asthma network. J Allergy Clin Immunol. 2014;133(1):27–33.
Benard B, Bastien V, Vinet B, Yang R, Kra**ovic M, Ducharme FM. Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice. Eur Respir J. 2017;50(2):1700148.
Ernst P, Ernst G. Neuropsychiatric adverse events of montelukast in children. Eur Respir J. 2017;50:1701020.
Acknowledgements
Medical writing assistance, in the form of the preparation and revision of the draft manuscript, was supported financially by Boehringer Ingelheim and provided by Rosie Robson of MediTech Media, under the authors’ conceptual direction and based on feedback from the authors.
Funding
This study was supported financially by Boehringer Ingelheim.
Author information
Authors and Affiliations
Contributions
The authors take full responsibility for the scope, direction, content of, and editorial decisions relating to the manuscript, were involved at all stages of development, and have approved the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
CV reports personal fees from Allergopharma, ALK, Bencard, Boehringer Ingelheim, Novartis, Stallergenes, Sanofi Avensis, Engelhard and DBV Technology, and grants from the German Society of Research (DFG), outside the submitted work. LG reports personal fees from Boehringer Ingelheim and serves as a speaker and member of the paediatric advisory board for Boehringer Ingelheim outside of the submitted work. AK reports personal fees from Boehringer Ingelheim, Covis, GlaxoSmithKline, Teva, Novartis, Pfizer, AstraZeneca, Purdue, Sanofi, Paladdin and Trudell outside the submitted work. AdlH is an employee of Boehringer Ingelheim. SG and EH have nothing to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vogelberg, C., Goldstein, S., Graham, L. et al. A comparison of tiotropium, long-acting β2-agonists and leukotriene receptor antagonists on lung function and exacerbations in paediatric patients with asthma. Respir Res 21, 19 (2020). https://doi.org/10.1186/s12931-020-1282-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-020-1282-9