Abstract
Background
Based on their anatomical location, rostral projections of nuclei are classified as ascending circuits, while caudal projections are classified as descending circuits. Upper brainstem neurons participate in complex information processing and specific sub-populations preferentially project to participating ascending or descending circuits. Cholinergic neurons in the upper brainstem have extensive collateralizations in both ascending and descending circuits; however, their single-cell projection patterns remain unclear because of the lack of comprehensive characterization of individual neurons.
Results
By combining fluorescent micro-optical sectional tomography with sparse labeling, we acquired a high-resolution whole-brain dataset of pontine-tegmental cholinergic neurons (PTCNs) and reconstructed their detailed morphology using semi-automatic reconstruction methods. As the main source of acetylcholine in some subcortical areas, individual PTCNs had abundant axons with lengths up to 60 cm and 5000 terminals and innervated multiple brain regions from the spinal cord to the cortex in both hemispheres. Based on various collaterals in the ascending and descending circuits, individual PTCNs were grouped into four subtypes. The morphology of cholinergic neurons in the pedunculopontine nucleus was more divergent, whereas the laterodorsal tegmental nucleus neurons contained richer axonal branches and dendrites. In the ascending circuits, individual PTCNs innervated the thalamus in three different patterns and projected to the cortex via two separate pathways. Moreover, PTCNs targeting the ventral tegmental area and substantia nigra had abundant collaterals in the pontine reticular nuclei, and these two circuits contributed oppositely to locomotion.
Conclusions
Our results suggest that individual PTCNs have abundant axons, and most project to various collaterals in the ascending and descending circuits simultaneously. They target regions with multiple patterns, such as the thalamus and cortex. These results provide a detailed organizational characterization of cholinergic neurons to understand the connexional logic of the upper brainstem.
Similar content being viewed by others
Background
The central nervous system has a highly ordered structure: external information is transmitted to a higher center through an ascending pathway, and decision-making information is delivered via a descending pathway to determine an individual’s physiological changes and behavior [1]. The upper brainstem (midbrain and pons), located between the diencephalon and medulla, has dense interconnections with different regions of the nervous system, from the spinal cord to the cortex [2, 3]. Previous studies have indicated that specialized populations of these neurons preferentially innervate the ascending and descending circuits. Dopamine (DA) neurons in the ventral tegmental area (VTA) [4] and different types of neurons in the dorsal raphe (DR) [5,6,7] send most of their axons to ascending circuits and a few to descending circuits. Two separate groups of glutamatergic neurons in the mesencephalic locomotor region (MLR) participate in different functions by projecting to the ascending and descending circuits, respectively [8]. Furthermore, cholinergic neurons in the pontine-tegmental cholinergic system, including the pedunculopontine nucleus (PPN) and laterodorsal tegmental nucleus (LDT), send abundant axons in both the ascending and descending circuits [9]; however, their projection patterns at the single-cell level have not been systematically characterized until now.
As the main cholinergic system in the rostral hindbrain [10], pontine-tegmental cholinergic neurons (PTCNs) send abundant axons along three major trajectories and are involved in various functions [11]. In the ascending dorsal circuits, PTCNs mediate prefrontal serotonin release from the DR [12] and participate in multiple functions, including auditory sensation [13], sensorimotor function [14], and spatial memory [15], by targeting different thalamic nuclei. Additionally, PTCNs innervate different neurons in the striatum (STR) and contribute to exploratory motor behaviors and action strategies [16]. In the ascending ventral circuits, previous studies have mainly focused on cholinergic modulation of the VTA and substantia nigra (SN), which are critically involved in mechanisms of reward [17], addiction [18], locomotion [23].
There are approximately 2000 PTCNs in one hemisphere of the mouse brain [24]. How does the pontine-tegmental cholinergic system innervate many brain regions and contribute to various functions with a limited number of neurons? Notably, the activation of PPN cholinergic neurons plays an opposite role in locomotion in the ascending and descending circuits [11]; however, the relationship between these two circuits is unclear. Although previous studies have acquired whole-brain projections of PTCNs [9], it is still unclear whether the ascending and descending axons belong to different groups or to the same group of neurons, which is urgently needed at the single-neuron level. Previous studies [25] have reconstructed partial axons in serial slices and provided preliminary evidence that individual PPN neurons have complex axonal projections. However, we lack the unabridged single-cell connection of the pontine-tegmental cholinergic system due to limited techniques for tracing and imaging.
In this study, we combined sparsely labeled fluorescence micro-optical sectioning tomography (fMOST) serial technologies [26, 27], and acquired a morphological atlas that may have uncovered the projection logic of PTCNs at the single-cell level.
Results
Sparse labeling and single-cell reconstruction
To obtain the fine morphology of individual cholinergic neurons, we employed a Cre-dependent virus for sparse labeling [28], the fMOST system for whole-brain imaging [26, 27], and GTree software for single-cell reconstruction [4], which mainly project to ascending circuits with a few axons projecting to descending circuits. However, a few of them have synchronous collaterals in the ascending and descending circuits [7]. Glutamatergic neurons in the PPN [38] and VTA [39] also have rich axons in both the ascending and descending circuits, but their single-cell projection patterns remain unknown. Moreover, the activation of PPN cholinergic neurons plays an opposite role in locomotion via the ascending and descending circuits [11], which means that these two circuits may belong to different groups. However, we found that all the reconstructed VTA/SN-projecting PPN cholinergic neurons had collaterals in the PRN. These results show that the opposite functions of the two cholinergic circuits may originate from different neurons or different responses of downstream neurons to the same neurons. Hence, PTCNs, which modulate many ascending and descending circuits, mainly originate from the same group of neurons.
On the other hand, we found that some neurons had richer collaterals in contralateral regions, which may have delivered more information to the contralateral hemisphere. The axial selection of ipsilateral and/or contralateral targets is crucial for integrating bilateral information and coordinated movements. PTCNs decrease significantly in patients with Parkinson’s disease (PD); characterized by abnormalities of movement, including tremors and difficulties with gait and balance [40]. The PPN is an important clinical target for deep brain stimulation in patients with PD [41], emphasizing the role that PTCNs may play in somatic homeostatic regulation by projecting abundant axons to bilateral hemispheres.
Projection logic of cholinergic axons ascending to the thalamus
The thalamus is an important relay station for sensorimotor information in mammals and acts as the main pathway for the pontine-tegmental cholinergic system to deliver information in ascending circuits. As the main source of acetylcholine for the thalamus, PTCNs project to the thalamus and form synaptic connections [9, 31]. In this study, we uncovered the logic of projection of the PTCNs to the thalamus at the single-cell level. Consistent with previous studies [42], most PTCNs directly innervate the thalamic nuclei. Individual PTCNs synchronously target multiple thalamic nuclei. We found that more than 80% of the reconstructed PTCNs innervated at least five thalamic nuclei, and some of them simultaneously projected to 18 nuclei in the bilateral thalamus. This indicates that the regulation of PTCNs in the thalamus is mainly diffuse, rather than concentrated in a few regions. The thalamic nuclei receive information from the whole brain and deliver it to diverse cortical areas [33, 34]. We speculate that individual PTCNs send complex information to various thalamic nuclei, which sort the information and then deliver it to specific cortical fields. The diverse functions of thalamic circuits projected from the PTCNs [13,14,15] may originate from the different responses of different thalamic nuclei.
Moreover, we decoded three projection patterns from PTCNs with different preferences in the ipsilateral and/or contralateral thalamus. There may be three different modes of information transmission from the pontine-tegmental cholinergic system to the thalamus for further information progress. Specifically, a large number of PTCNs targeted the bilateral thalamus and some preferred contralateral areas. These may play important roles in coordinated movement and contribute to some movement disorders diseases, such as PD [40]; the cholinergic circuits in the thalamus of these patients exhibit abnormal activity [14].
Morphological differences between the PPN and LDT cholinergic neurons
PPN and LDT cholinergic neurons are two components of the pontine-tegmental cholinergic system that have diverse characteristics with regard to connections and functions [9, 37]. They connect with similar regions with different preferences [9, 11, 31]. In this study, we demonstrated that the PPN and LDT cholinergic neurons have similar projection patterns with different preferences at the single-cell level. Comparatively, the PPN projected more divergent cholinergic axons to the ascending and descending circuits, whereas LDT neurons were more convergent. Various studies have suggested that the PPN and LDT cholinergic neurons target the same regions with different functions, such as the VTA [37] and SN [19]. The functional difference is related to the connectional difference between the two cholinergic nuclei: PPN neurons prefer motor nuclei, whereas LDT neurons prefer limbic nuclei [9, 11, 31]. Furthermore, single PTCNs have abundant and diverse co-projection axons that may contribute to functional differences. The thalamus is one of the main targets of PTCNs, and both individual PPN and LDT cholinergic neurons have rich collaterals in different thalamic nuclei with similar projection logic; however, they also show some differences [9, 31]. On the one hand, the PPN neurons had richer thalamic collaterals on average, and we found that some projected more than 50% of their axons to the thalamus. In contrast, the two groups of cholinergic neurons had different preferences for thalamic nuclei: PPN neurons preferred the thalamic motor nuclei, while LDT neurons preferred the limbic nuclei. In addition, the dendrites of the LDT cholinergic neurons had richer branches and longer dendrites than those of the PPN. As neuronal portals receive information, dendrites influence the number and identity of presynaptic inputs [43], which implies that LDT neurons may receive more afferent information.
Conclusions
Our study elucidated the single-cell morphology of PTCNs on a brain-wide scale and decoded the projection logic of the whole brain and specific circuits. We revealed that individual PTCNs send abundant axons in multiple nuclei in the ascending and descending circuits synchronously and can be grouped into four types with different projection patterns. Furthermore, we uncovered three different projection patterns from the PTCNs to the bilateral thalamus, and two separate pathways to the cortical areas. Our study mapped the axonal projections of the pontine-tegmental cholinergic system; thus providing researchers with a better understanding of its projection logic.
Methods
Animals
Adult (2–4 months) ChAT-ires-Cre mice were used in this study. ChAT-Cre transgenic mice (stock No: 018957) were purchased from Jackson Laboratory. The mice were kept under a condition of a 12-h light/dark cycle with food and water ad libitum. All animal experiments were approved by the Animal Care and Use Committee of Huazhong University of Science and Technology.
Tracer information
For sparse labeling, we employed the CSSP-YFP (5.2 × 1012 VG/mL) packed by Brain Case (Brain Case Co., Ltd., Shenzhen, China). CSSP-YFP virus is produced by co-packaging the rAAV-EF1α-DIO-Flp plasmid and rAAV-FDIO-EYFP plasmid with a ratio of 1:20,000 in the single rAAV production step [28].
Surgery and viral injection
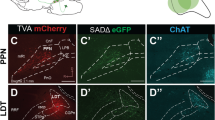
Before virus injection, we anesthetized the mice with mixed anesthetics (2% chloral hydrate and 10% ethyl urethane dissolved in 0.9% NaCl saline) according to their weight (0.1 ml/10 g). The brain of anesthetized mice was fixed with a stereotaxic holder to adjust the position of the skulls. Then a cranial drill (~ 0.5 mm diameter) was employed to uncover the skulls above the targets. For sparse labeling, we injected 100 nl CSSP-YFP into the unilateral PPN or LDT.
Histology and immunostaining
The histological operations followed previous studies [33]. Shortly, four weeks after AAV injection, anesthetized mice were perfused with 0.01 M PBS (Sigma-Aldrich, USA), followed by 2.5% sucrose and 4% paraformaldehyde (PFA, Sigma-Aldrich, USA) in 0.01 M PBS. Then, the brains were removed and post-fixed in 4% PFA solution overnight.
For immunofluorescent staining, some samples were sectioned in 50 μm coronal slices with the vibrating slicer (Leica 1200S). All sections containing PPN or LDT were selected to characterize the labeled neurons in inject site. These sections were blocked with 0.01 M PBS containing 5% (wt/vol) bovine serum albumin (BSA) and 0.3% Triton X-100 for 1 h at 37 °C. Then the sections were incubated with the primary antibodies (12h at 4 °C): anti-ChAT (1:500, goat, Sigma-Aldrich, AB144P). Then the sections were washed in PBS five times at room temperature. Next, these sections were incubated with the fluorophore-conjugated secondary antibody (1:500, Abcam: Alexa-Fluor 647, donkey anti-goat) for 2 h at room temperature. After rinsing with PBS, DAPI (1 ng/mL) was performed on stained sections for 5 min, and sections were finally mounted after washing. We acquired the stained information of sections with the confocal microscope (LSM 710, Zeiss, Jena, Germany).
Imaging and 3D visualization
For whole-brain imaging, virus-labeled samples were dehydrated with alcohol and embedded with resin. Then the whole brain datasets were acquired with the fMOST system. In short, we fixed the embedded sample on the base and acquired the image of the top surface with two fluorescent channels; the imaged tissue was subsequently removed by diamond wheel. Thus, we obtained the continuous whole-brain dataset layer by layer with high resolution (0.32 μm × 0.32 μm × 1 μm).
For 3D visualization and statistical analysis of whole-brain datasets, we registered the whole-brain datasets to the Allen CCFv3 [30]. The methods of registration have been described previously [44]. Briefly, we employed image preprocessing to correct uneven illumination and then remove background noise. The down-sampling data (the voxel resolution of 10 μm × 10 μm × 10 μm) was uploaded into Amira software (v6.1.1, FEI, Mérignac Cedex, France) to distinguish and extract regional features of anatomical invariants, including the outline of the brain, the ventricles, and the corpus striatum, etc. Next, the gray-level-based registration algorithm (SyN) was employed to register the extracted features. Basic operations including extraction of areas of interest, resampling, and maximum projection performed via Amira software and Fiji (NIH).
Morphological reconstruction of single neurons
83 neurons were reconstructed from six brains. For single-cell morphological analysis, we reconstructed the morphology of sparsely labeled neurons with semi-automatic methods following previous studies [29]. Briefly, we acquired the spatial coordinates of labeled somas in high-resolution data and transformed the data format of GFP-labeled data from TIFF to TDI type via Amira first.
Then the data block containing the given soma was loaded into GTree software and we assigned the soma as the initial point and marked all its axons with unfinished tags. Next, we selected one uncompleted fiber and traced it in the next block with automatic tracing. Then we checked the traced fiber and marked its branches with unfinished tags. We repeated the above procedure until the selected fiber was finished and then we reconstructed the remaining unfinished axons until all the axons were achieved. The reconstructed neurons were checked back-to-back by three persons. The tracing results were saved in SWC format. Meanwhile, we registered the PI-labeled data and the corresponding tracing results to the reference atlas with the methods mentioned above. Considering the distribution of axons and synaptic connection does not always correlate [31], we counted the terminal branches of reconstructed neurons to represent the connection between single cholinergic neurons and their targets.
Statistical information
To distinguish the axons of passage vs. terminal innervations, we take the ended axons as terminal axons while others as passage axons. For terminals quantification, the reconstructed neurons carried spatial information of all nodes that we could calculate the ended nodes based on registered single neurons to obtain the terminals in different regions. In addition, we employed the Amira software to quantify the length of dendrites and axons of individual neurons. Statistical graphs were generated using GraphPad Prism v.8.02 and Microsoft Excel (Office 2020). We employed GraphPad Prism v. 8.02 for the significance test, Neurolucida360 software for the polar histogram, and MATLAB (2017a) for the Sholl Analysis. We conducted one-way ANOVA followed by Tukey’s post hoc tests to compare the difference among four projections patterns of same nuclei while two-tailed t-tests to compare the difference between the PPN and LDT. The confidence level was set to 0.05 (P value) and all results were presented as mean ± SEM.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. Supplemental Information includes five figures and two tables and can be found additional files. The TB-sized raw data of sparse-labeling samples and 3D data of reconstructed neurons can be accessed at http://atlas.brainsmatics.org/a/zhao2206.
Abbreviations
- PPN:
-
Pedunculopontine nucleus
- LDT:
-
Laterodorsal tegmental nucleus
- PTCNs:
-
Pontine-tegmental cholinergic neurons
- CTXpl:
-
Cortical plate
- CTXsp:
-
Cortical subplate
- OLF:
-
Olfactory areas
- ORB:
-
Orbital area
- MOs:
-
Secondary motor area
- MOp:
-
Primary motor area
- mPFC:
-
Medial prefrontal cortex
- ECT:
-
Ectorhinal area
- PERI:
-
Perirhinal area
- TEa:
-
Temporal association areas
- AuD:
-
Auditory areas
- STR:
-
Striatum
- PAL:
-
Pallidum
- BF:
-
Basal forebrain
- RT:
-
Reticular nucleus of the thalamus
- VENT:
-
Ventral group of the dorsal thalamus
- GENd:
-
Geniculate group, dorsal thalamus
- MED:
-
Medial group of the dorsal thalamus
- LAT:
-
Lateral group of the dorsal thalamus
- ILM:
-
Intralaminar nuclei of the dorsal thalamus
- GENv:
-
Geniculate group, ventral thalamus
- EPI:
-
Epithalamus
- ATN:
-
Anterior group of the dorsal thalamus
- DORpm:
-
Thalamus, polymodal association cortex related
- DORsm:
-
Thalamus, sensory-motor cortex related
- PVR:
-
Periventricular region
- PVZ:
-
Periventricular zone
- LZ:
-
Hypothalamic lateral zone
- MEZ:
-
Hypothalamic medial zone
- MBmot:
-
Midbrain, motor related
- MBsen:
-
Midbrain, sensory related
- MBsta:
-
Midbrain, behavioral state related
- VTA:
-
Ventral tegmental area
- SN:
-
Substantia nigra
- P-mot:
-
Pons, motor related
- P-sat:
-
Pons, behavioral state related
- P-sen:
-
Pons, sensory related
- PRN:
-
Pontine reticular nucleus
- CB:
-
Cerebellum
- PFL:
-
Paraflocculus
- MY:
-
Medulla
- MY-sen:
-
Medulla, sensory related
- MY-sat:
-
Medulla, behavioral state related
- MY-mot:
-
Medulla, motor related
References
Luo L. Principles of Neurobiology. New York: Garland Science; 2015.
Swanson LW, Hahn JD, Sporns O. Subsystem macroarchitecture of the intrinsic midbrain neural network and its tectal and tegmental subnetworks. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(20):e2101869118.
Rahman M, Tadi P. Neuroanatomy, Pons. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022.
Beier KT, Steinberg EE, DeLoach KE, **. Cell. 2015;162(3):622–34.
Xu Z, Feng Z, Zhao M, Sun Q, Deng L, Jia X, Jiang T, Luo P, Chen W, Tudi A, et al. Whole-brain connectivity atlas of glutamatergic and GABAergic neurons in the mouse dorsal and median raphe nuclei. Elife. 2021;10: e65502.
Cardozo Pinto DF, Yang H, Pollak Dorocic I, de Jong JW, Han VJ, Peck JR, Zhu Y, Liu C, Beier KT, Smidt MP, et al. Characterization of transgenic mouse models targeting neuromodulatory systems reveals organizational principles of the dorsal raphe. Nat Commun. 2019;10(1):4633.
Ren J, Isakova A, Friedmann D, Zeng J, Grutzner SM, Pun A, Zhao GQ, Kolluru SS, Wang R, Lin R, et al. Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. Elife. 2019;8:e49424.
Ferreira-Pinto MJ, Kanodia H, Falasconi A, Sigrist M, Esposito MS, Arber S. Functional diversity for body actions in the mesencephalic locomotor region. Cell. 2021;184(17):4564-4578 e4518.
Zhao P, Wang H, Li A, Sun Q, Jiang T, Li X, Gong H. The Mesoscopic Connectome of the Cholinergic Pontomesencephalic Tegmentum. Front Neuroanat. 2022;16:843303.
Moreno-Bravo JA, Perez-Balaguer A, Martinez-Lopez JE, Aroca P, Puelles L, Martinez S, Puelles E. Role of Shh in the development of molecularly characterized tegmental nuclei in mouse rhombomere 1. Brain Struct Funct. 2014;219(3):777–92.
Mena-Segovia J, Bolam JP. Rethinking the pedunculopontine nucleus: from cellular organization to function. Neuron. 2017;94(1):7–18.
Kinoshita H, Nishitani N, Nagai Y, Andoh C, Asaoka N, Kawai H, Shibui N, Nagayasu K, Shirakawa H, Nakagawa T. Ketamine-induced prefrontal serotonin release is mediated by cholinergic neurons in the pedunculopontine tegmental nucleus. Int J Neuropsychopharmacol. 2018;21(3):305–10.
Luo F, Yan J. Sound-specific plasticity in the primary auditory cortex as induced by the cholinergic pedunculopontine tegmental nucleus. Eur J Neurosci. 2013;37(3):393–9.
Muller ML, Albin RL, Kotagal V, Koeppe RA, Scott PJ, Frey KA, Bohnen NI. Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain. 2013;136(Pt 11):3282–9.
Mitchell AS, Dalrymple-Alford JC, Christie MA. Spatial working memory and the brainstem cholinergic innervation to the anterior thalamus. J Neurosci. 2002;22(5):1922–8.
Dautan D, Huerta-Ocampo I, Gut NK, Valencia M, Kondabolu K, Kim Y, Gerdjikov TV, Mena-Segovia J. Cholinergic midbrain afferents modulate striatal circuits and shape encoding of action strategies. Nat Communctions. 2020;11(1):1739.
Zhang C, Liu X, Zhou P, Zhang J, He W, Yuan TF. Cholinergic tone in ventral tegmental area: Functional organization and behavioral implications. Neurochem Int. 2018;114:127–33.
Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 2009;30(7):1358–69.
**ao C, Cho JR, Zhou C, Treweek JB, Chan K, McKinney SL, Yang B, Gradinaru V. Cholinergic mesopontine signals govern locomotion and reward through dissociable midbrain pathways. Neuron. 2016;90(2):333–47.
Fernandez SP, Broussot L, Marti F, Contesse T, Mouska X, Soiza-Reilly M, Marie H, Faure P, Barik J. Mesopontine cholinergic inputs to midbrain dopamine neurons drive stress-induced depressive-like behaviors. Nat Commun. 2018;9(1):4449.
Azzopardi E, Louttit AG, DeOliveira C, Laviolette SR, Schmid S. The role of cholinergic midbrain neurons in startle and prepulse Inhibition. J Neurosci. 2018;38(41):8798–808.
Lima JD, Sobrinho CR, Falquetto B, Santos LK, Takakura AC, Mulkey DK, Moreira TS. Cholinergic neurons in the pedunculopontine tegmental nucleus modulate breathing in rats by direct projections to the retrotrapezoid nucleus. J Physiol. 2019;597(7):1919–34.
Chen D, Cheng X, Yang X, Zhang Y, He Z, Wang Q, Yao G, Liu X, Zeng S, Chen J, et al. Map** the Brain-Wide Cholinergic Neurons Projecting to Skeletal Muscle in Mice by High-Throughput Light Sheet Tomography. Neurosci Bull. 2021;37(2):267–70.
Li X, Yu B, Sun Q, Zhang Y, Ren M, Zhang X, Li A, Yuan J, Madisen L, Luo Q, et al. Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc Natl Acad Sci USA. 2018;115(2):415–20.
Mena-Segovia J, Sims HM, Magill PJ, Bolam JP. Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J Physiol. 2008;586(12):2947–60.
Gong H, Xu D, Yuan J, Li X, Guo C, Peng J, Li Y, Schwarz LA, Li A, Hu B, et al. High-throughput dual-colour precision imaging for brain-wide connectome with cytoarchitectonic landmarks at the cellular level. Nat Commun. 2016;7:12142.
Zhong Q, Li A, ** R, Zhang D, Li X, Jia X, Ding Z, Luo P, Zhou C, Jiang C, et al. High-definition imaging using line-illumination modulation microscopy. Nat Methods. 2021;18(3):309–15.
Sun P, ** S, Tao S, Wang J, Li A, Li N, Wu Y, Kuang J, Liu Y, Wang L, et al. Highly efficient and super-bright neurocircuit tracing using vector mixing-based virus cocktail. bioRxiv. 2020:705772.
Zhou H, Li S, Li A, Huang Q, **ong F, Li N, Han J, Kang H, Chen Y, Li Y, et al. GTree: an Open-source Tool for Dense Reconstruction of Brain-wide Neuronal Population. Neuroinformatics. 2020;19(2):305-17.
Wang Q, Ding S-L, Li Y, Royall J, Feng D, Lesnar P, Graddis N, Naeemi M, Facer B, Ho A, et al. The allen mouse brain common coordinate framework: A 3D reference atlas. Cell. 2020;181(4):936-953.e920.
Huerta-Ocampo I, Hacioglu-Bay H, Dautan D, Mena-Segovia J. Distribution of Midbrain Cholinergic Axons in the Thalamus. eNeuro. 2020;7(1):ENEURO.0454-19.
Baksa B, Kovács A, Bayasgalan T, Szentesi P, Kőszeghy Á, Szücs P, Pál B. Characterization of functional subgroups among genetically identified cholinergic neurons in the pedunculopontine nucleus. Cell Mol Life Sci. 2019;76(14):2799–815.
Zhao P, Zhao M, Wang H, Jiang T, Jia X, Tian J, Li A, Gong H, Li X. Long-range inputome of cortical neurons containing corticotropin-releasing hormone. Sci Rep. 2020;10(1):12209.
Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508(7495):207–14.
Valencia M, Chavez M, Artieda J, Bolam JP, Mena-Segovia J. Abnormal functional connectivity between motor cortex and pedunculopontine nucleus following chronic dopamine depletion. J Neurophysiol. 2013;111(2):434–40.
Luo F, Liu X, Wang C, Yan J. The pedunculopontine tegmental nucleus: a second cholinergic source for frequency-specific auditory plasticity. J Neurophysiol. 2011;105(1):107–16.
Dautan D, Souza AS, Huerta-Ocampo I, Valencia M, Assous M, Witten IB, Deisseroth K, Tepper JM, Bolam JP, Gerdjikov TV, et al. Segregated cholinergic transmission modulates dopamine neurons integrated in distinct functional circuits. Nat Neurosci. 2016;19(8):1025–33.
Dautan D, Kovacs A, Bayasgalan T, Diaz-Acevedo MA, Pal B, Mena-Segovia J. Modulation of motor behavior by the mesencephalic locomotor region. Cell Rep. 2021;36(8): 109594.
An S, Li X, Deng L, Zhao P, Ding Z, Han Y, Luo Y, Liu X, Li A, Luo Q, et al. A Whole-Brain Connectivity Map of VTA and SNc Glutamatergic and GABAergic Neurons in Mice. Front Neuroanatomy. 2021;15: 818242.
Mazzoni P, Shabbott B, Cortés JC. Motor control abnormalities in Parkinson’s disease. Cold Spring Harbor Perspect Med. 2012;2(6): a009282.
Nowacki A, Galati S, Ai-Schlaeppi J, Bassetti C, Kaelin A, Pollo C. Pedunculopontine nucleus: an integrative view with implications on Deep Brain Stimulation. Neurobiol Dis. 2019;128:75–85.
Sofroniew MV, Priestley JV, Consolazione A, Eckenstein F, Cuello AC. Cholinergic projections from the midbrain and pons to the thalamus in the rat, identified by combined retrograde tracing and choline acetyltransferase immunohistochemistry. Brain Res. 1985;329(1):213–23.
Lefebvre JL, Sanes JR, Kay JN. Development of dendritic form and function. Annu Rev Cell Dev Biol. 2015;31:741–77.
Ni H, Tan C, Feng Z, Chen S, Zhang Z, Li W, Guan Y, Gong H, Luo Q, Li A. A robust image registration interface for large volume brain atlas. Sci Rep. 2020;10(1):2139.
Acknowledgements
We thank Yang Yang and Mengting Zhao from Huazhong University of Science and Technology for their help with experiments and data analysis. We thank the Optical Bioimaging Core Facility of HUST for support with data acquisition.
Funding
This work was supported by STI2030-Major Projects (No.2021ZD0201001), NSFC projects (Nos. 61890953, 32192412, 31871088), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-014), and Sichuan Science and Technology Program (2023NSFSC1575) and the Director Fund of WNLO.
Author information
Authors and Affiliations
Contributions
XL and HG conceived and designed the study. PZ performed the experiments and analyzed the data. TJ acquired continuous whole-brain datasets. HW, XJ, and AL processed the whole-brain data and reconstructed the neurons. XL, HG, and PZ wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Animal Care and Use Committee at Huazhong University of Science and Technology (IACUC Number: S799).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Fig. S1. Sparse labeling of cholinergic neurons.Sparsely labeled 3D continuous datasets.Typicalinject site of the PPN and LDT.Labeled fibers rangefrom the cortical areas to the spinal cord. Scale bar,1000μm;200μm;100μm. Fig. S2. 3D view of all 83reconstructed cholinergic neurons. Pentagons presented neurons had richer axonbranches in the contralateral hemisphere. Red triangle pointed neuronrestricted its fibers in the ipsilateral thalamus. Green triangle-pointedneurons sent richer fibers to the contralateral thalamus. Fig. S3. PFL-projectionneurons and the soma of reconstructed neurons.3D view of three neurons projecting to thePFL.3Dview of all reconstructed soma. Fig. S4.Morphology and polar analysis of 83 reconstructed neurons. Fig. S5. Quantitative analysisof axons of thalamic projection PTCNs.the proportion of thalamic terminals in individualneurons. Two-sided t-tests.The distribution of axonal terminals in the thalamus of singleneurons. Each column displayed one neuron. Boxes in different colors explainedthe number of terminals of a single neuron in different brain regions.The number of mainthalamic targets of individual PTCNs.The ratio ofterminals in the ipsilateral thalamus of single neurons. Green dots showedneurons confined their axons in the ipsilateral thalamus. Purple-filled dotsrepresent neurons that had richer axons in the contralateral thalamus. Bluedots represent neurons that did not target the thalamus. For the details of abbreviations for brainregions see Nomenclature and abbreviations. For detailed statistics of thalamicterminals, see Additional file 3: Table 2.
Additional file 2:
Table 1. Quantification of reconstruction neurons.
Additional file 3:
Table 2. Quantification of axonal terminals in the thalamus.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, P., Jiang, T., Wang, H. et al. Upper brainstem cholinergic neurons project to ascending and descending circuits. BMC Biol 21, 135 (2023). https://doi.org/10.1186/s12915-023-01625-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12915-023-01625-y