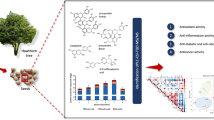
Abstract
Background
Fabaceae plays a crucial role in African traditional medicine as a source of large number of important folk medication, agriculture and food plants. In a search of potential antioxidant and anti-inflammatory candidates derived from locally cultivated plants, the flowers of Tipuana tipu (Benth.) Lillo growing in Egypt were subjected to extensive biological and phytochemical studies. The impact of the extraction technique on the estimated biological activities was investigated.
Methods
The flowers were extracted using different solvents (aqueous, methanol, water/methanol (1:1), methanol/methylene chloride (1:1), and methylene chloride). The different extracts were subjected to antioxidant (DPPH, ABTS, and FRAP) and anti-inflammatory (COX-2 and 5-LOX) assays. The methanol extract was assessed for its inhibitory activity against iNOS, NO production, and pro-inflammatory cytokines (NF-KB, TNF-R2, TNF-α, IL-1β, and IL-6) in LPS-activated RAW 264.7 macrophages. The composition-activity relationship of the active methanol extract was further investigated using a comprehensive LC–QTOF-MS/MS analysis. The major identified phenolic compounds were further quantified using HPLC-DAD technique. The affinity of representative compounds to iNOS, COX-2, and 5-LOX target active sites was investigated using molecular docking and molecular dynamics simulations.
Results
The methanol extract exhibited the highest radical scavenging capacity and enzyme inhibitory activities against COX-2 and 5-LOX enzymes with IC50 values of 10.6 ± 0.4 and 14.4 ± 1.0 µg/mL, respectively. It also inhibited iNOS enzyme activity, suppressed NO production, and decreased the secretion of pro-inflammatory cytokines. In total, 62 compounds were identified in the extract including flavonoids, coumarins, organic, phenolic, and fatty acids. Among them 18 phenolic compounds were quantified by HPLC-DAD. The highest docking scores were achieved by kaempferol-3-glucoside and orientin. Additionally, molecular dynamics simulations supported the docking findings.
Conclusion
The flower could be considered a potentially valuable component in herbal medicines owing to its unique composition and promising bioactivities. These findings encourage increased propagation of T. tipu or even tissue culturing of its flowers for bioprospecting of novel anti-inflammatory drugs. Such applications could be adopted as future approaches that benefit the biomedical field.
Similar content being viewed by others
Background
Family Fabaceae is the third largest flowering plants family, exceeded only by the Orchidaceae and Compositae [1]. It comprises about 730 genera and more than 19,400 species. Plants of this family grow in a variety of habitats and climates throughout the world. Many are important food and agricultural plants, including Glycyrrhiza glabra (licorice), Glycine max (soybean), Medicago sativa (alfalfa), Phaseolus (beans) and Pisum sativum (pea) [2]. The Genus Tipuana (Benth.) Benth. (Subfamily Papilionoideae) is represented by Tipuana tipu (Benth.) Lillo species, a South African tree. Also, it is commonly grown in several other countries, such as Egypt, Australia, Argentina, Uruguay, Paraguay, Brazil, and Bolivia [3, 4]. It is an ornamental tree and is also used as a supplementary food to ruminants (e.g., sheep), mostly the leaves which showed high nutritive value [5]. T. tipu was recommended for wound healing, hemorrhoids, gastrointestinal tract disorders, and abdominal and rheumatic pains. It has anti-inflammatory and free-radical scavenging activities, in addition it is used as an astringent for its high tannin content [6, 7]. Ethyl acetate and methylene chloride extracts of the aerial parts of T. tipu were proved to be efficient in inhibiting Listeria monocytogenes and Staphylococcus aureus growth. Moreover, leaf extracts showed promising nephroprotective and antimalarial activities [8]. Few phytochemical studies were performed on the leaf and the bark of T. tipu isolating formononetin, β-sitosterol, β-sitosterol glucoside, lupeol, α-amyrin, 1-nonadecanol, alpinumisoflavone, protocatechualdehyde, protocatechuic acid, and stearic acid [3, 5, 9, 10]. A kaempferol glycoside with marked anti-inflammatory activity, kaempferol 3-O-α-L-rhamnopyranosyl-(1→6)-O-[β-D-glucopyranosyl-(1→2)-4-O-acetyl-α-L-rhamnopyranosyl-(1→2)]-β-D-galactopyranoside, has been isolated from T. tipu (Benth.) Lillo leaves growing in Egypt, with another three flavonol glycosides, kaempferol 3-O-rutinoside, rutin, and kaempferol-3-O-[α-L-rhamnopyranosyl-(1→6)]-[α-L-rhamnopyranosyl-(1→2]-β-glucopyranoside [11].
Despite all progress in synthetic biotechnology and chemistry, herbs are still indispensable sources of medicinal preparations and considered as an important part of healthcare throughout the world [12]. In addition, plant-based medications have emerged as a promising source for develo** new pharmaceuticals and functional items due to the side effects of synthetics [13, 14], along with their higher safety margin and lower cost [15]. Surveying the current literature, all studies mainly focused on the leaves of T. tipu (Benth.) Lillo. Although flowers are considered as rich sources for valuable constituents depending on our phytochemical investigations, few and incomplete studies were traced concerning their contents and antioxidant and anti-inflammatory bioactivities [16]. Accordingly, different solvent extracts of the flowers (aqueous, methanolic, water/methanol (1:1), methanol/methylene chloride (1:1), and methylene chloride) were tested for their antioxidant capacities using different methods, such as DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2’-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)), and FRAP (ferric reducing antioxidant power). Furthermore, the in vitro anti-inflammatory (COX-2 and 5-LOX) effects of all extracts were evaluated. Then, the most active extract (methanolic extract) was evaluated for its inhibitory activity against the inducible nitric oxide synthase enzyme (iNOS), nitric oxide production (NO), and the secretion of the pro-inflammatory cytokines (NF-KB, TNF-R2, TNF-α, IL-1β, and IL-6) in LPS-activated RAW 264.7 macrophages. As well as it was of a great interest to provide a comprehensive metabolic profiling of T. tipu flowers using an integrated approach of LC-QTOF-MS/MS and HPLC-DAD analyses. Further, computational study using molecular docking simulations with iNOS, COX-2, and 5-LOX active sites was performed to establish for the first time the correlation between the elucidated chemical profiles and the anti-inflammatory effects of flowers. Thus, this study aims to provide a holistic understanding of the phytochemical composition of T. tipu flowers in relation to their biological potential, as the literature lacks such detailed information. Such an approach is required for future breeding programs alongside quality control purposes that offer chemical-based evidence regarding T. tipu biological potential and health benefits.
Materials and methods
The detailed procedures are described in the Supplementary online resource.
Plant material
Flowers of Tipuana tipu (Benth.) Lillo were collected during Spring season (March 2022) from the Zoo (Giza, Egypt). The taxonomic identity of the plant material was kindly authenticated and verified by Mrs. Teresa Labib, Head of the Taxonomists at Orman Botanic Garden, Egypt. This study complies with local, national, and international guidelines, and no specific consent was required for the collection of the plant material.
Preparation of different flower extracts
The flowers (1.5 kg) were air-dried, grinded, and extracted separately in triplicates (500 g each) with water, methanol, water/methanol (1:1), methanol/methylene chloride (1:1), and methylene chloride (twice, 3 L each) by cold maceration. The extracts were then dried under vacuum in a rotary evaporator at 50 ºC and the residues were kept separately in tight containers until analysis. Finally, the dried residues (30 mg each) were dissolved separately in DMSO for the biological assays and the dried methanolic residue (10 mg) was dissolved in methanol for LC-MS analysis. Three biological replicates were prepared for each solvent type and extracted in parallel under the same conditions (n = 3).
LC/QTOF-MS/MS analysis
T. tipu flowers methanolic extract was subjected to metabolic analysis using a 6530 Q-TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA) outfitted with an autosampler (G7129A), a quaternary pump (G7104C). The extract was separated on a Zorbax RP-18 column (150 mm × 3 mm, dp = 2.7 μm, Agilent Technologies). The flow rate was 0.2 mL/min, and the injection volume was 2 µL. Water (A) and acetonitrile (B) with formic acid (0.1%) in each utilized as the solvents and applied using a gradient elution as follows 5% B linearly increased to 90% B within 30 min, then remained isocratic at 100% B for 2 min before linearly decreasing back to 5% B for the following 5 min. Mass spectra were obtained using ESI in negative ionization mode using the conditions previously described [17]. Briefly, the Q-TOF mass spectrometer was optimized in negative ESI mode with the following parameters: capillary voltage 3500 V, drying gas temperature and flow rate 350 °C and 8 L/min, respectively, nebulizer gas 30 psi, fragmentor voltage 150 V, skimmer voltage 60 V, and OCT 1 RF Vpp voltage 300 V. The auto-MS/MS mode was used in the m/z range of 50-2000, with an MS scan rate of 10 spectra/s and an MS/MS scan rate of 6 spectra/s. The precursor selection criteria were as follows: maximum precursor per cycle 9, absolute threshold 200 counts, relative threshold 0.01%, purity stringency 100%, purity cutoff 30%, precursors sorted by abundance only, and isolation window ∼ 1.3 m/z (narrow). Mass Hunter software (ver. B.06, Agilent Technologies, Inc. 2012) was used for instrument control, data acquisition, and processing of the MS/MS spectra. Metabolites were characterized by their retention times (min), exact molecular ion mass (M-H)–, MS/MS fragment ions relative to accessible databases, such as the Human Metabolome Database (http://www.hmdb.ca/) and lipidMaps (https://www.lipidmaps.org/).
Results and discussion
Antioxidant and inhibitory activities against COX-2 and 5-LOX of different flower extracts
The pharmaceutical industry is transitioning towards nature-derived antioxidants because of the adverse effects associated with synthetic drugs [18]. The capability of various natural constituents to reduce inflammation is supposed to be from the following: firstly, acting as antioxidants; then, interfering with the signaling of free radical species; finally, decreasing the pro-inflammatory signaling transductions [19]. Therefore, the antioxidant (ABTS, FRAP, and DPPH methods), as well as inhibitory activities against COX-2 and 5-LOX of aqueous, methanol, 50% aqueous methanol, methylene chloride, and 50% methylene chloride/methanol extracts of the flowers were assessed using in vitro assays (Fig. 1), in an attempt to understand the ability of these extracts to develop effective intervention for inflammatory disease prevention strategies. Herein, the methanolic extract exhibited the highest antioxidant potential equivalent to 181.5 ± 0.8 µM TE/g (DPPH), 261.1 ± 1.1 µM TE/g (ABTS), and 270.3 ± 2.5 µM TE/g (FRAP), in comparison to ascorbic acid (standard drug). Relative to the other extracts, the methanol extract showed the highest inhibition against COX-2 (IC50 10.6 ± 0.4 µg/mL) compared to Celecoxib (IC50 1.70 ± 0.0 µg/mL), and the highest 5-LOX inhibitory potential (IC50 14.4 ± 1.0 µg/mL) compared to Zileuton as a standard (IC50 5.65 ± 0.4 µg/mL).
Aq: aqueous extract; H2O/ME: water/methanol (1:1); MC: methylene chloride extract; ME: methanolic extract; ME/MC: methanol/methylene chloride (1:1) extract. Data are represented as mean ± standard deviation of three replicates. Different letters on the bar imply significant differences at P < 0.0001 with Tukey’s test.
Considering the fact that the methanol extract exhibited the highest radical scavenging potential and enzyme inhibitory activities, it is important to further ascertain its anti-inflammatory behavior. Therefore, its inhibitory effects against iNOS enzyme, NO production, and pro-inflammatory cytokines secretion (TNF-α, IL-1β, IL-6, NF-KB, and TNF-R2) in LPS-activated RAW 264.7 macrophages were evaluated.
Cell viability on RAW264.7 macrophages
In MTT assay, T. tipu flowers methanolic extract (ME) did not show cytotoxic activity (up to 500 µg/mL) (Fig. S1) when assayed on RAW264.7 macrophages (after 24 h incubation), indicating ideal safety profile of the methanolic extract so it can be used in alleviating painful disease symptoms and improving human health.
Inhibitory activity of methanolic extract (ME) of T. tipu flowers against iNOS enzyme activity, NO production, and secretion of pro-inflammatory cytokines in LPS stimulated RAW 264.7 macrophages
The excess production of inflammatory mediators in many ailments, like asthma, arthritis, vascular disease, obesity, and dermatitis has become one of the first global morbidity causes [20]. Inflammatory mediators, such as nitric oxide (NO) and pro-inflammatory cytokines [tumor necrosis factor-α (TNF-α), interleukin (IL)- IL-6, and IL-1β] are produced by lipopolysaccharide (LPS, a gram-negative bacteria) through activation of surface receptors, such as tumor necrosis factor receptors (TNF-R2), several protein kinases (MAPKs; extracellular signal-regulated kinase [ERK] and c-Jun N-terminal kinase [JNK]), and transcriptional factors as nuclear factor-κB (NF-κB) in macrophages [21]. In addition, LPS-stimulated macrophages exhibit up-regulation of iNOS expression through the generation of inflammatory cytokines. Therefore, inhibition of iNOS, inflammatory mediators and proinflammatory cytokines in LPS-stimulated macrophages can provide an effective approach for prevention of inflammatory disorders [22].
The ME of T. tipu flowers inhibited iNOS in LPS-induced RAW264.7 macrophages, with IC50 value of 11.1 ± 1.0 µM relative to parthenolide as a standard drug (IC50 2.2 ± 0.0 µM). The results also indicated that the ME of the flowers is an effective inhibitor of LPS-induced NO production in RAW 264.7 cells and decreased the release of TNF-α, IL-β, IL-6, NF-KB, and TNF-R2, compared to standard drugs (Table 1; Fig. 2). These findings imply that ME of the flowers might be utilized as a natural anti-inflammatory resource. This prompted the use of LC-MS metabolic profiling of the ME to enable the preliminary identification of key components that may contribute synergistically to the anti-inflammatory and antioxidant effects, followed by their quantification and verification using computational analyses that could explain the structure-activity relationships.
The inhibitory effect of methanolic extract (ME) of T. tipu flowers on pro-inflammatory cytokines (TNF-α, IL-β, and IL-6) production in LPS stimulated RAW 264.7 macrophages. ME: methanolic extract. * Significant from negative control at P < 0.0001. # Significant from positive control at P < 0.0001 with Tukey’s test
Metabolite profiling
The deficit of reports concerning the chemical profiles of the flowers under investigation motivated the performance of this study to investigate the chemical composition of T. tipu flowers through a non-targeted metabolite profiling of the prepared ME for detecting and identifying large numbers of metabolites using ultra performance liquid chromatography (UPLC) coupled with mass spectrometry (MS). Sixty-two compounds have been tentatively identified in the methanolic extract of T. tipu flowers after careful inspection of MS/MS data, comparison with on line database and reported literature [11, 23]. The compounds belonged to various classes encompassing: 2 sugars, 6 amino acids, 6 organic acids, 14 phenolic acids, 2 coumarins, 12 flavonoids, 8 fatty acids, and 12 phospholipids. Details of the tentatively identified compounds, including retention times, m/z of the detected molecular ions, fragment ions, and molecular formulas, as well as putative identifications are tabulated in Table 2. The base peak chromatogram of the analyzed extract in the negative ionization mode is shown in Fig S2. (supplementary materials). The representative MS/MS spectra of selected compounds from each class are displayed in Figs. S3-11. Interestingly, the main identified compounds were phenolic acids and flavonoids (a total of 26), agreeing with the previous reports for T. tipu leaves [11, 24].
Identification of phenolic acids
Phenolic compounds contribute significantly to antioxidant activity [25]. Fourteen phenolic acids belonged to various classes have been identified from their exact masses and fragmentation patterns [23]. They were mainly benzoic and hydroxycinnamic acid derivatives and were eluted after organic acids in the chromatographic separation. In the MS/MS spectra of phenolic acids, the loss of CO2 group from the carboxylic acid moiety (-44 amu), loss of CO group (-28 amu), as well as the loss of water molecule (-18 amu) led to the formation of characteristic fragment ions [26]. Five free benzoic acids have been identified i.e., benzoic acid, hydroxy benzoic acid, methoxy benzoic acid, protocatechuic acid, and vanillic acid. Two benzoic acid glycosides, protocatechuic acid glucoside and vanillic acid glucoside, were also detected with molecular ions at m/z 315.0721 and 329.0876, respectively, and MS2 spectra due to loss of glucose moiety (-162 amu) and CO2 group. Seven hydroxycinnamic acids were identified caffeic acid glucoside, p-coumaric acid glucoside, chlorogenic acid, cinnamic acid, ferulic acid, coumaric acid pentoside, and dihydrocaffeic acid glucoside. Cinnamic and ferulic acids produced molecular ions (M-H)– at m/z 147.0655 and 193.0494, respectively, and MS2 spectra due to removal of CO2 group from the carboxylic acid function (at m/z 103 and 149, respectively). Chlorogenic acid showed a molecular ion peak at m/z 353.0873 (C16H17O9–) and a base peak at m/z 191 corresponding to the deprotonated quinic acid. Two glycosides of coumaric acid have been identified with molecular ions at m/z 295.0456 (C13H11O8–) and 325.0914 (C7H13O7–). Both showed main fragment ions at m/z 163 and 119 of coumaric acid and its decarboxylated form. Thus, they were identified as coumaric acid pentoside (Fig. S3) and coumaric acid glucoside (Fig. S4), respectively. Caffeic acid glucoside and dihydrocaffeic acid glucoside have been identified from their molecular ions at m/z 341.0879 and 343.1021, respectively. In their MS/MS spectra, they produced fragment ions due to loss of the sugar moiety to give the free acids at m/z 179 and 181, respectively, besides other characteristic ions due to sequential losses of CO, CO2, and H2O groups. The methanol extract demonstrated high phenolic acids content suggesting its antioxidant potential. Additionally, phenolic acids were found to have anti-inflammatory and protective effects against many oxidative stress related diseases viz. cancers, diabetic and cardiovascular disorders [27, 28]. Because of their phenol moiety and resonance-stabilized structure, phenolic acids have antioxidant properties due to electron and H-atom donations and radical quenching mechanisms [27, 29, 30].
Identification of coumarins
Esculetin and its glycoside esculin have been detected in the T. tipu extract for the first time. They displayed molecular ion peaks at m/z 177.0128 and 339.0724, respectively. Esculetin (Fig. S5) showed typical fragmentation pattern of coumarins including successive losses of CO2 (-44 amu) and CO (-28 amu) groups from the molecular ion to produce three main fragment ions at m/z 133 [(M-H)-44]–, 105 [(M-H)-44-28]–, and 89 [(M-H)-44*2]– [31]. While esculin (Fig. S6) gave its base peak at m/z 177 due to the breakdown of the glycosidic linkage and removal of the glucose moiety (-162 amu). Several authors have pointed to the varied range of pharmacological effects of esculetin and esculin, including antioxidant, anti-inflammatory, anticancer, antidiabetic, and neuroprotective [49]. The average SASA values for the complete frames of the Apo-COX protein, kaempferol-3-glucoside-COX, and orientin-COX complex systems were 23,437, 23,156, and 23243.86, respectively (Fig. 3D). 27152Å, 26451Å, and 26796.51Å, for Apo-LOX protein, kaempferol-3-glucoside-LOX, orientin–LOX complex systems, respectively (Fig. 4D). 20645Å, 20053.87Å, and 20125.54Å for Apo-NOS protein, kaempferol-3-glucoside-NOS, and orientin–NOS complex systems, respectively (Fig. 5D).
When paired with the data from the RMSD, RMSF, and ROG computations, the SASA finding revealed that the kaempferol-3-glucoside and orientin complex systems remain intact inside the catalytic binding site for the three enzymes.
[A] RMSD of the protein backbone’s Cα atoms. [B] RMSF of each residue of the protein backbone Cα atoms of protein residues (C) ROG of Cα atoms of protein residues; (D) solvent accessible surface area (SASA) of the Cα of the backbone atoms relative (black) to the starting minimized over 20 ns for the catalytic domain binding site of cyclooxygenase-2 enzyme with kaempferol-3-glucoside complex system (red), and orientin complex system (blue)
[A] RMSD of the protein backbone’s Cα atoms. [B] RMSF of each protein residue’s Cα atom; (c) ROG of each residue’s Cα atom; (d) solvent accessible surface area (SASA) of the backbone atoms relative to the starting minimized over 20 ns for the catalytic domain binding site of 5-lipoxygenase with kaempferol-3-glucoside complex system (red), and orientin complex system (blue)
[A] RMSD of the protein backbone’s Cα atoms. [B] RMSF of each residue of the protein backbone Cα atoms of protein residues (c) ROG of Cα atoms of protein residues; (d) solvent accessible surface area (SASA) of the Cα of the backbone atoms relative (black) to the starting minimized over 20 ns for the catalytic domain binding site of Human Nitric oxide synthase with kaempferol-3-glucoside complex system (red), and orientin complex system (blue)
Binding interaction mechanism based on binding free energy calculation
A well-known method for determining the free binding energies of small molecules to biological macromolecules is the molecular mechanics energy methodology (MM/GBSA), which combines the generalized Born and surface area continuum solvation [50]. The MM-GBSA program in AMBER18 was used to determine the binding free energies using snapshots taken from the systems’ trajectories. Except for Gsolv, all of the estimated energy components (Table 5) showed large negative values indicating favorable interactions.
Identification of the critical residues responsible for ligands binding
To understand more about key residues involved in the inhibition of the catalytic binding site of COX-2 receptor, the total energy involved when substance contacts these enzymes was further broken down into the participation of specific site residues. From Fig. 6A, the major favorable contribution of kaempferol-3-glucoside to the COX-2 binding site receptor is mainly observed from residues Val57 (-0.154 kcal/mol), Hie58 (-0.463 kcal/mol), Val85 (-1.362 kcal/mol), Leu86 (-0.799 kcal/mol), Arg89 (-0.308 kcal/mol), Gln161 (-1.095 kcal/mol), Ile314 (-0.456 kcal/mol), Tyr317 (-0.24 kcal/mol), Val318 (-2.078 kcal/mol), Leu321 (-1.975 kcal/mol), Ser322 (-1.165 kcal/mol), Tyr 324 (-1.19 kcal/mol), Leu328 (-0.432 kcal/mol), Leu353 (-0.288 kcal/mol), Tyr354 (-1.295 kcal/mol), Ile486 (-0.356 kcal/mol), Phe 487 (-1.81 kcal/mol), Val492 (-2.61 kcal/mol), Gly495 (-1.245 kcal/mol), and Ala496 (-2.36 kcal/mol).
Regarding orientin, the major favorable contribution to the COX-2 binding site receptor is principally observed from residues Hie58 (-0.267 kcal/mol), Met82 (-0.311 kcal/mol), Val 85 (-1.239 kcal/mol), Ile314 (-0.341 kcal/mol), Tyr317 (-0.455 kcal/mol), Val318 (-2.468 kcal/mol), Leu321 (-1.68 kcal/mol), Ser 322 (-2.189 kcal/mol), Tyr324 (-2.357 kcal/mol), Leu328 (-1.015 kcal/mol), Phe350 (-0.348 kcal/mol), Leu353 (-0.427 kcal/mol), Tyr354 (-1.535 kcal/mol), Trp356 (-0.418 kcal/mol), Val492 (-2.354 kcal/mol), Gly495 (-1.08 kcal/mol), Ala496 (-1.794 kcal/mol), and Ser499 (-2.752 kcal/mol) (Fig. 6B).
From Fig. 7A, the major favorable contribution of kaempferol-3-glucoside to the 5-lipoxygenase binding site receptor is predominantly observed from residues Trp141 (-0.652 kcal/mol), Phe145 (-0.387 kcal/mol), Phe 302 (-1.255 kcal/mol), Gln306 ( -2.856 kcal/mol), Thr307 (-0.975 kcal/mol), Hid310 (-0.588 kcal/mol), Leu311 (-2.367 kcal/mol), Hip315 (-1.015 kcal/mol), Ala353 (-1.003 kcal/mol), Arg354 (-0.6 kcal/mol), Leu357 (-2.475 kcal/mol), Ile358 (-1.68 kcal/mol), Gly359 (-0.552 kcal/mol), Hie361 (-0.766 kcal/mol), Pro 498 (-0.405 kcal/mol), Trp528 (-0.637 kcal/mol), Hie529 (-2.488 kcal/mol), Ala532 (-1.369 kcal/mol), Val533 (-0.78 kcal/mol), and Leu356 (-1.372 kcal/mol). Alternatively, the major favorable contribution of orientin to the 5-lipoxygenase binding site receptor is mostly detected from residues Phe302 (-1.036 kcal/mol), Gln306 (-3.975 kcal/mol), Thr307 (-1.105 kcal/mol), Hid310 (-2.021 kcal/mol), Leu311 (-0.822 kcal/mol), Ala353 (-1.617 kcal/mol), Gln356 (-0.585 kcal/mol), Hid479 (-0.338 kcal/mol), Asn483 (-1.139 kcal/mol), Gln486 (-3.03 kcal/mol), Trp528 (-0.507 kcal/mol), Ala532 (-1.011 kcal/mol), Val533 (-1.265 kcal/mol), Leu536 (-3.171 kcal/mol), and Ile602 (-5.123 kcal/mol) (Fig. 7B).
Finally, from Fig. 8A, the major favorable contribution of kaempferol-3-glucoside to the Human Nitric oxide synthase (NOS) synthesize enzymes protein binding site receptor is predominantly observed from residues Met39 (-0.616 kcal/mol), Arg118 (-2.059 kcal/mol), Cys119 (-0.312 kcal/mol), Ile120 (-0.745 kcal/mol), Met293 (-0.613 kcal/mol), Glu296 (-1.238 kcal/mol), Arg300 (-0.455 kcal/mol), Asp301 (-2.298 kcal/mol), Ile381 (-1.47 kcal/mol), Trp382 (-4.6 kcal/mol), Leu383 (-0.527 kcal/mol), and Pro386 (-0.741 kcal/mol). On the other, the major favorable contribution of orientin to the Human Nitric oxide synthase (NOS) protein binding site receptor is predominantly observed from residues Ser37 (-0.468 kcal/mol), Met39 (-2.975 kcal/mol), Gln182 (-2.593 kcal/mol), Asp199 (-0.709 kcal/mol), Trp265 (-0.422 kcal/mol), Tyr266 (-0.782 kcal/mol), Pro269 (-0.259 kcal/mol), Tyr292 (-0.716 kcal/mol), Met293 (-0.249 kcal/mol), Glu296 (-2.435 kcal/mol), Ile297 (-0.42 kcal/mol), Arg300 (-1.125 kcal/mol), Asp301 (-4.969 kcal/mol), Asp304 (-0.265 kcal/mol), Arg307 (-0.26 kcal/mol), Trp382 (-3.375 kcal/mol), and Pro386 (-0.896 kcal/mol) (Fig. 8B). Interestingly, the docking results come in agreement with the previous reports demonstrated the antioxidant and anti-inflammatory activities of orientin and kaempferol-3-glucoside [51, 52].
Conclusion
This investigation revealed the promising efficiency of T. tipu flowers as antioxidant and anti-inflammatory drug, which is obviously related to its chemical profile and as indicated by the computational analysis. The results suggested the useful incorporation of the flowers’ extract in pharmaceutical and cosmetic industries owing to its potential to suppress the inflammatory key enzymes (iNOS, COX-2, and 5-LOX), as well as scavenge free radicals through its high antioxidant properties. However, it should be noted that these findings are preliminary, and further research with the methanol extract or after purification of specific compounds, followed by more detailed and conclusive in vivo and clinical studies, is strongly advised to fully exploit T. tipu flowers’ potential in the pharmaceutical industry.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Abbreviations
- 5-LOX:
-
5-lipoxygenase
- ABTS, 2,2':
-
azino-di(3-ethylbenzthiazoline-6-sulfonic acid
- COX-2:
-
cyclooxygenase 2
- DPPH, 2,2:
-
diphenyl-1-picrylhydrazyl
- FRAP:
-
ferric reducing antioxidant power
- IC50 :
-
half maximal inhibitory concentration
- IL:
-
interleukin; iNOS: inducible nitric oxide synthase
- LC-QTOF-MS/MS:
-
liquid chromatography-quadrupole-time-of-flight-mass spectrometry
- LPS:
-
lipopolysaccharide
- MD:
-
molecular dynamic
- ME:
-
methanolic extract
- MTT:
-
(3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide)
- NF-KB:
-
Nuclear factor kappa B
- NO:
-
nitric oxide
- TE:
-
Trolox equivalent
- TNF-α:
-
tumor necrosis factor alpha
- TNF-R2:
-
tumor necrosis factor receptor 2
References
Harborne JB, Boulter D, Turner BL. Chemotaxonomy of the Legumin-Osae. London, UK: Academic Press Inc.(London) Ltd.; 1971.
Rahman A, Parvin MIAJP. Taxonomic studies on the family Fabaceae (weeds) at Rajshahi University campus. Plant. 2015;3:20–5.
Amen YM, Marzouk AM, Zaghloul MG, et al. Bioactive compounds from Tipuana tipu growing in Egypt. J Am Sci. 2013;9:334–9. https://doi.org/10.7537/marsjas091013.44.
Trudgen MS, Scott JK, Lambers H et al. Identifying limitations for invasion: the effect of phosphorus availability on the growth of the non-native tree, Tipuana tipu. Aust J Bot. 2023.
Norton B, Waterfall M. The nutritive value of Tipuana tipu and Calliandra Calothyrsus as supplements to low-quality straw for goats. Small Rumin Res. 2000;38:175–82.
Quiroga R, Meneses L, Bussmann RW. Medicinal ethnobotany in huacareta (Chuquisaca, Bolivia). J Ethnobiol Ethnomed. 2012;8:1–14.
Barboza GE, Cantero JJ, Núñez C, et al. Medicinal plants: a general review and a phytochemical and ethnopharmacological screening of the native Argentine Flora. Kurtziana. 2009;34:7–365.
El Ayeb-Zakhama A, Sakka‐Rouis L, Bergaoui A, et al. Chemical composition and allelopathic potential of essential oils from Tipuana tipu (Benth.) Kuntze cultivated in Tunisia. Chem Biodivers. 2016;13:309–18. https://doi.org/10.1002/cbdv.201500083.
De Braga A, Gottlieb O, Leite de Almeida M. Extractives of [stemwood and roots of] Tipuana tipu.(Chemistry of Brazilian Leguminosae XXXI). Phytochemistry. 1971;10:2552–3.
Lucía A, Claudia M, Sara S, et al. Antibacterial activity of extracts obtained from Senna corymbosa and Tipuana tipu. PhOL. 2012;3:158–61.
Amen YM, Marzouk AM, Zaghloul MG, et al. A new acylated flavonoid tetraglycoside with anti-inflammatory activity from Tipuana tipu leaves. Nat Prod Res. 2015;29:511–7.
Bursal E, Yılmaz MA, Izol E, et al. Enzyme inhibitory function and phytochemical profile of Inula discoidea using in vitro and in silico methods. Biophys Chem. 2021;277:106629. https://doi.org/10.1016/j.bpc.2021.106629.
Saleem H, Zengin G, Sarfraz M, et al. Phytochemical composition and in-vitro pharmacological evaluation of Emex australis Steinh: a natural source of enzyme inhibitors. S Afr J Bot. 2021;143:374–81. https://doi.org/10.1016/j.sajb.2021.02.023.
Saleem H, Zengin G, Locatelli M, et al. Investigation of phytochemical composition and enzyme inhibitory potential of Anagallis arvensis L. Nat Prod Res. 2022;36:3750–5. https://doi.org/10.1080/14786419.2021.1880404.
Gill MS, Saleem H, Ahemad N. Plant extracts and their secondary metabolites as modulators of kinases. Curr Top Med Chem. 2020;20:1093–104. https://doi.org/10.2174/1568026620666200224100219.
Kansoh AL, Afifi MS, Elgindi OD, et al. Chemical composition, antimicrobial and cytotoxic activities of essential oil and lipoidal matter of the flowers and pods of Tipuana tipu growing in Egypt. Can J Pure Appl Sci. 2009;3:661–8.
Ads EN, Hassan SI, Rajendrasozhan S, et al. Isolation, structure elucidation and antimicrobial evaluation of natural pentacyclic triterpenoids and phytochemical investigation of different fractions of Ziziphus spina-christi (L.) stem bark using LCHRMS analysis. Molecules. 2022;27:1805.
Saleem H, Zengin G, Locatelli M, et al. Filago germanica (L.) Huds. Bioactive constituents: secondary metabolites fingerprinting and in vitro biological assays. Ind Crop Prod. 2020;152:112505. https://doi.org/10.1016/j.indcrop.2020.112505.
Ibrahim RM, Abdel-Baki PM, Elmasry GF, et al. Combinative effects of akarkara root-derived metabolites on anti-inflammatory and anti-alzheimer key enzymes: integrating bioassay-guided fractionation, GC-MS analysis, and in silico studies. BMC Complement Med Ther. 2023;23:413. https://doi.org/10.1186/s12906-023-04210-6.
Kim K-N, Ko S-C, Ye B-R, et al. 5-Bromo-2-hydroxy-4-methyl-benzaldehyde inhibited LPS-induced production of pro-inflammatory mediators through the inactivation of ERK, p38, and NF-κB pathways in RAW 264.7 macrophages. Chem Biol Interact. 2016;258:108–14.
Kim E-A, Kim S-Y, Kim J, et al. Tuberatolide B isolated from Sargassum macrocarpum inhibited LPS-stimulated inflammatory response via MAPKs and NF-κB signaling pathway in RAW264. 7 cells and zebrafish model. J Funct Foods. 2019;52:109–15.
Yoon W-J, Lee NH, Hyun C-G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J Oleo Sci. 2010;59:415–21.
Ibrahim RM, El-Halawany AM, Saleh DO, et al. HPLC-DAD-MS/MS profiling of phenolics from Securigera securidaca flowers and its anti-hyperglycemic and anti-hyperlipidemic activities. RevBbras Farmacogn. 2015;25:134–41.
Afifi MS, Elgindi OD, Bakr RO. Flavonoids with acetylated branched glycans and bioactivity of Tipuana tipu (Benth.) Kuntze leaf extract. Nat Prod Res. 2014;28:257–64.
Saleem H, Zengin G, Locatelli M, et al. Pharmacological, phytochemical and in-vivo toxicological perspectives of a xero-halophyte medicinal plant: Zaleya pentandra (L.) Jeffrey. Food Chem Toxicol. 2019;131:110535. https://doi.org/10.1016/j.fct.2019.05.043.
Hamed AR, El-Hawary SS, Ibrahim RM, et al. Identification of chemopreventive components from halophytes belonging to Aizoaceae and Cactaceae through LC/MS—Bioassay guided approach. J Chromatogr Sci. 2021;59:618–26.
Mahdy NE, Abdel-Baki PM, El-Rashedy AA et al. Modulatory Effect of Pyrus pyrifolia Fruit and its phenolics on key enzymes against metabolic syndrome: Bioassay-Guided Approach, HPLC Analysis, and in Silico Study. Plant Foods Hum Nutr. 2023; 1–7.
Karagecili H, Yılmaz MA, Ertürk A, et al. Comprehensive metabolite profiling of Berdav propolis using LC-MS/MS: determination of antioxidant, anticholinergic, antiglaucoma, and antidiabetic effects. Molecules. 2023;28:1739. https://doi.org/10.3390/molecules28041739.
Zengin G, Mahomoodally MF, Aktumsek A, et al. Functional constituents of six wild edible Silene species: a focus on their phytochemical profiles and bioactive properties. Food Biosci. 2018;23:75–82. https://doi.org/10.1016/j.fbio.2018.03.010.
Ak G, Zengin G, Sinan KI, et al. A comparative bio-evaluation and chemical profiles of Calendula officinalis L. extracts prepared via different extraction techniques. Appl Sci. 2020;10:5920. https://doi.org/10.3390/app10175920.
Yang W, Ye M, Liu M, et al. A practical strategy for the characterization of coumarins in Radix Glehniae by liquid chromatography coupled with triple quadrupole-linear ion trap mass spectrometry. J Chromatogr A. 2010;1217:4587–600.
Zhang L, **e Q, Li X, Esculetin. A review of its pharmacology and pharmacokinetics. Phytother Res. 2022;36:279–98.
Sharifi-Rad J, Cruz-Martins N, López-Jornet P et al. Natural coumarins: exploring the pharmacological complexity and underlying molecular mechanisms. Oxid Med Cell Longev. 2021; 2021.
Witaicenis A, Seito LN, da Silveira Chagas A, et al. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine. 2014;21:240–6.
Bouhafsoun A, Yilmaz MA, Boukeloua A, et al. Simultaneous quantification of phenolic acids and flavonoids in Chamaerops humilis L. using LC–ESI-MS/MS. Food Sci Technol. 2018;38:242–7. https://doi.org/10.1590/fst.19917.
Saleem H, Yaqub A, Rafique R, et al. Nutritional and medicinal plants as potential sources of enzyme inhibitors toward the bioactive functional foods: an updated review. Crit Rev Food Sci Nutr. 2023;1–24. https://doi.org/10.1080/10408398.2023.2217264.
Spagnuolo C, Moccia S, Russo GL. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem. 2018;153:105–15.
Zhang M, Duan C, Zang Y, et al. The flavonoid composition of flavedo and juice from the pummelo cultivar (Citrus grandis (L.) Osbeck) and the grapefruit cultivar (Citrus paradisi) from China. Food Chem. 2011;129:1530–6.
Adamczak A, Ożarowski M, Karpiński TM. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J Clin Med. 2019;9:109.
Hounsome N, Hounsome B, Lobo M. Biochemistry of Vegetables: Major Classes of Primary Metabolites (Carbohydrates, Amino Acids, Vitamins, Organic Acids, and Fatty Acids). Handbook of Vegetables and Vegetable Processing. 2018; 25–46.
Okba MM, Abdel-Baki PM, Abu-Elghait M, et al. UPLC-ESI-MS/MS profiling of the underground parts of common Iris species in relation to their anti-virulence activities against Staphylococcus aureus. J Ethnopharmacol. 2022;282:114658.
Mauerhofer C, Philippova M, Oskolkova OV, et al. Hormetic and anti-inflammatory properties of oxidized phospholipids. Mol Aspects Med. 2016;49:78–90.
Hospital A, Goñi JR, Orozco M, et al. Molecular dynamics simulations: advances and applications. Adv Appl Bioinforma Chem. 2015;37–47. https://doi.org/10.2147/AABC.S70333.
Mirzaei S, Eisvand F, Hadizadeh F, et al. Design, synthesis and biological evaluation of novel 5, 6, 7-trimethoxy-N-aryl-2-styrylquinolin-4-amines as potential anticancer agents and tubulin polymerization inhibitors. Bioorg Chem. 2020;98:103711. https://doi.org/10.1016/j.bioorg.2020.103711.
Hasanin M, Hashem AH, El-Rashedy AA, et al. Synthesis of novel heterocyclic compounds based on dialdehyde cellulose: characterization, antimicrobial, antitumor activity, molecular dynamics simulation and target identification. Cellulose. 2021;28:8355–74. https://doi.org/10.1007/s10570-021-04063-7.
Machaba KE, Mhlongo NN, Soliman ME. Induced mutation proves a potential target for TB therapy: a molecular dynamics study on LprG. Cell Biochem Biophys. 2018;76:345–56. https://doi.org/10.1007/s12013-018-0852-7.
Wijffels G, Dalrymple B, Kongsuwan K, et al. Conservation of eubacterial replicases. IUBMB Life. 2005;57:413–9. https://doi.org/10.1080/15216540500138246.
Pan L, Patterson JC. Molecular dynamics study of zn (aβ) and zn (aβ) 2. PLoS ONE. 2013;8:e70681.
Richmond TJ. Solvent accessible surface area and excluded volume in proteins: Analytical equations for overlap** spheres and implications for the hydrophobic effect. J Mol Biol. 1984;178:63–89. https://doi.org/10.1016/0022-2836(84)90231-6.
Cournia Z, Allen B, Sherman W. Relative binding free energy calculations in drug discovery: recent advances and practical considerations. J Chem Inf Model. 2017;57:2911–37. https://doi.org/10.1021/acs.jcim.7b00564.
Taherkhani A, Khodadadi P, Samie L et al. Flavonoids as Strong Inhibitors of MAPK3: A Computational Drug Discovery Approach. Int J Anal Chem. 2023; 2023.
Essa AF, Teleb M, El-Kersh DM et al. Natural acylated flavonoids: their chemistry and biological merits in context to molecular docking studies. Phytochem Rev,. 2022; 1–40.
Funding
This research received no external funding.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
R.M.I.: Conceptualization, Formal analysis, Data curation, Writing – original draft. P.M.A.: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft. A.A.E.: Methodology, Formal analysis. N.E.M.: Conceptualization, Formal analysis, Data curation, Writing – original draft. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ibrahim, R.M., Abdel-Baki, P.M., El-Rashedy, A.A. et al. LC-MS/MS profiling of Tipuana tipu flower, HPLC-DAD quantification of its bioactive components, and interrelationships with antioxidant, and anti-inflammatory activity: in vitro and in silico approaches. BMC Complement Med Ther 24, 176 (2024). https://doi.org/10.1186/s12906-024-04467-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-024-04467-5