Abstract
Background
Juvenile idiopathic arthritis (JIA), an autoimmune disease affecting children or adolescents and causing joint or systemic symptoms, reportedly has a negative effect on the patients’ body height. This study aimed to identify factors attributable to substantially reduced adult height (SRAH) in JIA patients.
Methods
This single-center retrospective cohort study included patients from 2009 to 2019 in Taiwan. We collected JIA patients aged > 18 years at enrollment with a definite diagnosis and undergoing regular outpatient clinic follow-up or disease remission. Target height difference (THD), defined by adult height minus mid-parental height, was calculated for each patient. The calculation results yielded two groups, of which positive THD was defined as the optimal height (OH group) and those with THD below two standardized deviations as the SRAH group. Descriptive statistics and logistic regression analysis were used to analyze the data.
Results
Of 92 JIA patients, 57 and 12 were in the OH and the SRAH groups. Earlier disease onset, especially before the six-year-old, was noted in the SRAH group (p = 0.026). The distribution of JIA subtypes differed significantly between the two groups (p < 0.001); enthesis-related arthritis was the commonest subtype in the OH group, and systemic JIA was the commonest in the SRAH group. Half of the patients in the SRAH group had an active disease status at enrollment, which was higher than the OH group (50.0% vs. 21.1%, p = 0.066). More patients in the SRAH group had received orthopedic surgery due to JIA (25% vs. 3.5%, p = 0.034). Multiple logistic regression analysis showed that SRAH was independently related to systemic JIA (OR = 37.6, 95%CI 1.2-1210.5; p = 0.041).
Conclusion
The subtype of systemic JIA, with its characteristics of early disease onset and active disease status, was the essential factor that significantly impacted adult height.
Similar content being viewed by others
Background
Juvenile idiopathic arthritis (JIA), the most common cause of chronic arthritis in childhood, affects specific articular joints and results in arthritis and constitutional symptoms in adolescents or children below 16 years old. JIA is classified into seven subtypes based on onset age, number of joint involvements, associated symptoms, and serological examinations of each patient [1]. A short body height in adulthood is usually a main concern in JIA patients and their parents. The estimated prevalence of short stature in JIA ranges from around 10–40% among different JIA subtypes [2, 3]. In addition, a short body height is also more prevalent in polyarticular and systemic subtypes of JIA, with prevalence rates ranging from 10.4% in polyarticular JIA and 41.0% in systemic JIA (sJIA) [3, 4].
The possible mechanisms of JIA-related short body height are complicated, including the inflammatory process of the disease, poor nutrition, growth hormone deficiency, and the effects of treatments, according to previous data [4,5,6]. Persistent inflammation plays a critical role. Previous studies revealed that pro-inflammatory cytokines negatively affected growth plate chondrogenesis [7]. The most prominent inflammatory cytokines involved in the pathogenesis of sJIA, including TNFα, interleukin-1, and interleukin-6, can influence molecular signaling pathways, chondrocyte, osteoclast, and osteoblast activities and aggravate joint inflammation [7,8,9,10,11,12]. Silencing of cytokine effects results in more stable height growth in JIA patients; however, overactivation of these cytokines might result in poor longitudinal growth [13]. Aside from the cytokines production due to active disease, several studies revealed the influence of some medications on the height of JIA patients, such as glucocorticoids (GC) and tumor necrosis factor (TNF)-α inhibitors. Wang et al. reported that using GC for more than one year may significantly impair body height growth [14]. The TNF-α inhibitor Etanercept was found to have the potential to improve body height after introducing biological agents in recent years [15, 16]. Although many studies mentioned the factors affecting growth velocity and height percentile in childhood, they seldom discussed final adult height (AH) in JIA. In this study, we aimed to identify the factors that may contribute to substantially reduced adult height (SRAH) in patients with JIA.
Methods
Study populations
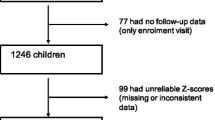
We collected data from patients diagnosed with JIA between 2009 and 2019 at the National Taiwan University Children’s Hospital. JIA was diagnosed according to the International League of Associations for Rheumatology (ILAR) criteria [1]. Moreover, patients should be 18 years old upon enrollment and reach their AH while enrolled in our study. Those who were under regular follow-ups until disease remission were included. We excluded patients who belonged to the unspecified JIA type, lost follow-up, and died during the outpatient clinic follow-up until remission during the disease (Fig. 1).
Flowchart of patient enrollment. JIA, juvenile idiopathic arthritis; AH, adult height, defined as current adult height; MPH, midparental height, defined as mean of parental height ± 6.5 cm; THD, target height difference, AH minus MPH; OH group, optimal height group, whose AH – MPH > 0 cm; SARH group, substantially reduced height group, whose AH – MPH <–5 cm; SD, standard deviation
Measurement of height
At the clinic visit, we collected anthropometric data, including height (cm) and weight (kg). Height was measured by trained nurses using the same wall-mounted stadiometer. The parents’ heights were obtained at the clinic if available or from their self-measurements at home. We calculated the midparental height (MPH) by adding 6.5 cm to the mean of the parental height in males or by subtracting 6.5 cm in females. The differences between AH and MPH, the target height difference (THD) in this study, were calculated to minimize the inherent differences in each patient. Those patients with positive THDs belonged to the optimal height (OH) group. Patients whose THDs were below two standard deviations of the general population were defined as the substantially reduced height (SRAH) group. The definition of SRAH conformed to the clinical definition of short stature [17]. According to a previous study, the two standard deviations in the normal population were approximately 5 cm [18]. Significant factors for SRAH were compared and analyzed among the OH and SRAH groups.
Baseline data collection
We reviewed each patient’s clinical characteristics, laboratory reports, therapeutic regimens, disease activity, and treatment response. The clinical features included age, sex, body mass index (BMI) at enrollment, age at disease onset, and JIA subtype. At initial diagnosis, laboratory data such as white blood cell count, hemoglobin level, platelet count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level were recorded. Therapeutic regimens were collected and analyzed, such as disease-modifying antirheumatic drugs (DMARDs), methotrexate, systemic GC, and TNFα inhibitors. The administration route of systemic GC was oral prednisolone or intravenous methylprednisolone, with an equivalent accumulation dosage of prednisolone (mg/kg/day) recorded in cases who used GC for over a month. We also recorded the use of intra-articular corticosteroid injections (IACIs). The disease activity and treatment response were classified as active disease or remission (on/off medication), according to the preliminary criteria from Wallace 2004 [19]. Inactive disease is defined as the absence of clinical symptoms such as arthritis, arthralgia or uveitis, normal ESR, and CRP level, and no GC requirement at the last clinic visit. Remission on medication was based on the definition of inactive disease for more than six consecutive months, even under any treatment. Remission off medication was defined as an inactive disease status for at least 12 continuous months without any medication to treat arthritis or uveitis.
Statistical analyses
For quantitative and ordinal data, patient data were expressed as counts, percentages, or medians with interquartile ranges (IQRs). Statistical analyses were performed using SAS software (version 9.4, SAS Institute Inc., Cary, USA). The Chi-square and Fisher’s exact tests were used to compare categorical variables. Differences between continuous variables were compared using an independent t-test. The Kruskal–Wallis rank test was used for nonparametric analysis within groups. Univariate and multiple logistic regression models were used to identify independent risk factors of SRAH development in JIA patients. We chose factors with a p-value less than 0.01 in the multiple logistic regression model with gender adjustment and checked the collinearity. Statistical significance was set at p < 0.05.
Results
Patients’ characteristics
A total of 92 patients were included in this study. The flowchart of patient enrollment is described in Fig. 1, and the height distribution based on their TH and MPD is in Fig. 2. We excluded those with THD between 0 cm and − 5 cm and enrolled the remaining 69 patients in the analysis, and there were 57 (82.6%) and 12 (17.4%) patients in the OH and SRAH groups, respectively. Their characteristics are listed in Table 1. The enrolled age was 22.2 years old in the OH group and 23.2 years old in the SRAH group. We observed a trend of earlier onset age in the SRAH group (9.6 vs. 11.7 years old, p = 0.20), especially before six years old, compared with the OH group (33.3% vs. 7.0%, p = 0.026). Males were predominant in both groups. The median (IQR) THD was 4.7 (3.1) cm in the OH group and − 9.0 (2.3) cm in the SRAH group. The distribution of JIA subtypes significantly differed between the two groups (p < 0.001). ERA was the most common subtype in the OH group, followed by the polyarticular type. However, the systemic type was the most common in the SRAH group. Initial laboratory data collected at the diagnosis were compared as listed in Table 1. No differences in other laboratory data were noted between the OH and SRAH groups, including antinuclear antibody (ANA), human leukocyte antigen (HLA)-B27, rheumatoid factor (RF) positivity, hemogram, or inflammatory markers.
The treatment and disease statuses of enrolled patients are summarized in Table 2. In our study, GC remained one of the main treatments for active or severe disease activity. Around half of the patients in both groups had received GC for over one month. Most patients in both groups used GC for less than six months. Among the patients who used GC over one month, the average accumulation GC dose, equivalent to the prednisolone dosage, was 0.26 mg/kg/day, ranging from 0.01 to 0.77 mg/kg/day. Nine cases had received IACIs. Half the patients in the SRAH group had received GC for more than 12 months compared to the 23.3% in the OH group. All patients had received DMARDs, and methotrexate was frequently used. Though the difference did not reach significance, more patients in the SRAH group (91.7%) had received TNFα inhibitors and used them for a longer duration, with a mean time of 10.1 years, compared to the data in the OH group. As for the long-term treatment response and disease activity, more patients in the SRAH group still had an active disease status (50.0% vs. 21.1%, p = 0.066) at enrollment. Most patients (78.9%) in the OH group achieved inactive disease, 73.7% reached remission, and 17.5% did not take any medication. By comparison, in the SRAH group, 41.7% reached remission, but none were medication-free. Five patients had received orthopedic surgery due to complications of JIA. Four patients belonged to sJIA, and another one had ERA. Three were in the SRAH group, and the ratio was significantly higher than in the OH group (25% vs. 3.5%, p = 0.034).
Risk factors associated with SRAH in JIA patients
We conducted univariate logistic regression analyses to evaluate the risk factors of SRAH in JIA patients, as shown in Table 3. The onset age remained one of the factors of SRAH, especially in patients with earlier disease onset below six years old (OR = 6.63, 95%CI = 1.37–31.93, p = 0.018). The systemic subtype was significantly related to SRAH (OR = 42.5, 95%CI = 4.83–373.41, p = < 0.001), compared to the ERA subtype as the reference group. None of the laboratory data at the diagnosis and treatment course were factors related to SRAH. The patients with active disease status at enrollment had a significantly positive correlation with SRAH (OR = 4.2, 95%CI = 1.09–16.19, p = 0.037). History of orthopedic surgery due to complications of JIA was significantly related to SRAH (OR = 9.17, 95%CI = 1.34–62.71, p = 0.024). After adjustment, sJIA remained the risk factor of SRAH (OR = 37.6, 95%CI 1.2-1210.5; p = 0.041).
Subgroup analysis by gender
To eliminate the effects on growth in different genders, we performed a subgroup analysis according to the gender of each patient (Supplementary Table 1). Among the 52 male patients, 44 were in the OH group and 8 in the SRAH group. The JIA subtypes showed significant differences between the OH and SRAH groups (p = 0.025). ERA was the most common subtype in the OH group (65.9%); however, all subtypes were evenly distributed in the SRAH group. Besides, the disease status in both groups differed (p = 0.038), with most of the OH group achieving remission status (79.5%) and half of the patients in the SRAH group still in active disease.
There were 17 female patients, including 13 in the OH group and 4 in the SRAH group. The patients in the SRAH group had significantly earlier disease onset age than those in the OH group (4.3 vs. 12.0 years old, p = 0.032). A significantly lower BMI was found in the SRAH group (17.9\(\pm\)1.8 vs. 21.9\(\pm\)4.4 kg/m2, p=0.015). The distribution of the JIA subtype in female patients showed similar results in the total population, with more ERA (38.5%) and polyarticular (38.5%) subtypes in the OH group and primarily systemic (75%) subtype in the SRAH group (p = 0.016). Two initial laboratory parameters, ESR and WBC count, were significantly higher in the SRAH group. Also, patients in the SRAH group required longer treatment durations of TNFα inhibitors (15.1\(\pm\)1.6 vs. 6.3\(\pm\)7.9 years, p = 0.049).
Discussion
In this single-center cohort study, we analyzed the factors affecting JIA patients’ final AH, including basic characteristics, laboratory reports, treatments, and disease status. We found sJIA subtype, an earlier disease onset, mainly before six years old, a history of JIA-associated orthopedic surgery, and active disease status during follow-ups were correlated with SRAH in JIA patients. This study may be the first one to discuss SRAH in JIA patients by comparing the final and estimated AH based on the MPH.
Since chronic inflammation, the essential feature of JIA, is often related to growth disorders ranging from a mild decrease in growth velocity to severe growth impairment, many studies assessed the growth in JIA patients and the factors associated with growth restriction or short stature. The previous studies regarding growth in JIA used z-scores as a height parameter and for the definition of short stature compared to the general population. Our study used the THD based on the patient’s MPH to minimize the genetic effects of their parent’s height. We reported that 13.0% of JIA patients had SRAH, with 71.4% in children with sJIA, 23.1% in children with the oligoarticular disease, 15.3% in children with the polyarticular disease, and only 0.5% in children with ERA. Because ERA remains the predominant JIA subtype in Taiwan based on previous epidemiologic studies, the rate of SRAH is relatively lower in our JIA patients [20].
Our study found that patients with sJIA accounted for most of the SRAH group (41.7%), and sJIA was the most essential predicting factor for SRAH in our results. McErlane et al. reported that patients with sJIA had higher rates of severe growth restriction and lower height growth velocity among all JIA subtypes [21]. Barut et al. reported a 15-year and single-center experience of the clinical features and outcomes in 168 sJIA patients. Growth retardation was recorded in 11.3% of them, especially in those patients with persistent or polycyclic clinical courses [22]. Systemic JIA was characterized by its significant systemic inflammation, earlier disease onset, longer disease course, and poor disease outcome [23]. Several studies supported evidence that the inflammatory process may promote growth hormone resistance and disease [8, 11]. The findings of association between persistent inflammation and growth indicate that sJIA patients may experience reduced height in adulthood than other subtypes due to profound inflammatory processes in this group of patients.
A variety of drugs have been found to affect the AH in JIA. Glucocorticoids, useful anti-inflammatory drugs, were often used in JIA patients with flared-up diseases and were usually prescribed a few decades ago. Wang et al. reported the negative influence of GC on AH in JIA patients, according to the data collected from 1973 to 1995 at the same medical institute in this study [14]. However, we did not find significant differences between the OH and SRAH groups regarding the duration of GC use. The main difference may be that 79.3% of the JIA patients enrolled were diagnosed after 2003, when the first biologic for JIA was introduced in our hospital, and patients had more treatment options like DMARDs than GC. Only a few studies have reported a correlation between DMARDs and final AH. An open-label, nonrandomized trial in 2010 showed a significant increase in the mean height percentiles when methotrexate (MTX) and Etanercept were combined in polyarticular or sJIA after three years of follow-up [24]. This report suggested that multiple medication use may improve the inflammatory process and indirectly decrease body height. Further subgroup analysis may be required to demonstrate the effect of treatment in different JIA subgroups.
In our study, orthopedic surgery was significantly related to SRAH. Previous cohort studies showed that 10% of patients with JIA needed orthopedic surgeries, such as total hip and knee arthroplasty, to correct their bone deformity or joint contracture [25, 26]. Our study found that all JIA patients with SRAH and previous operation history had an active disease condition at enrollment. This result informed us that those with active disease and poor treatment response might require orthopedic surgery, and these populations may also experience short AH due to their severe disease status.
This study had some limitations. First, this was a retrospective cohort study with a recall and selection bias. However, we enrolled those patients with continuous follow-ups and collected the primary outcomes objectively to minimize bias. Second, our study lacked serial disease severity scores to clarify the effect of long-term disease severity on AH. However, we used the latest disease status to understand the relationships between JIA disease control and SRAH. Lastly, the case number was insufficient to perform a subgroup analysis of the JIA subtypes. The data presented here could provide important information about AH in the post-biologics era of JIA. However, more detailed prospective records and evaluations of different JIA subtypes are still needed in the future.
Conclusion
Reduced AH is one of the main concerns in patients with JIA. In this real-world experience, we identified factors such as systemic subtype, younger disease onset age, active disease status, and history of JIA-related orthopedic surgery that were correlated with SRAH in patients with JIA. Among them, sJIA was the main factor affecting AH. For JIA patients with these characteristics, clinicians might need to be aware of their growth problems during the disease course and provide intervention if signs of growth retardation present.
Data availability
All data generated or analyzed during this study were included in this manuscript.
Abbreviations
- AH:
-
Adult height
- ANA:
-
Antinuclear antibody
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- DMARDs:
-
Disease-modifying antirheumatic drugs
- ERA:
-
Enthesis-related arthritis
- ESR:
-
Erythrocyte sedimentation rate
- HLA:
-
Human leukocyte antigen
- ILAR:
-
International League of Associations for Rheumatology
- JIA:
-
Juvenile idiopathic arthritis
- OH:
-
Optimal height
- MPH:
-
Midparental height
- RH:
-
Reduced height
- SRAH:
-
Substantially reduced adult height
- THD:
-
Target height difference
- TNF:
-
Tumor necrosis factor
References
Giancane G, Consolaro A, Lanni S, Davì S, Schiappapietra B, Ravelli A. Juvenile idiopathic arthritis: diagnosis and treatment. Rheumatol Ther. 2016;3(2):187–207.
Minden K. Adult outcomes of patients with juvenile idiopathic arthritis. Horm Res. 2009;72(Suppl 1):20–5.
Simon D, Fernando C, Czernichow P, Prieur AM. Linear growth and final height in patients with systemic juvenile idiopathic arthritis treated with longterm glucocorticoids. J Rheumatol. 2002;29(6):1296–300.
Simon D, Lucidarme N, Prieur AM, Ruiz JC, Czernichow P. Treatment of growth failure in juvenile chronic arthritis. Horm Res. 2002;58(Suppl 1):28–32.
Wong SC, MacRae VE, Gracie JA, McInnes IB, Galea P, Gardner-Medwin J, et al. Inflammatory cytokines in juvenile idiopathic arthritis: effects on physical growth and the insulin-like-growth factor axis. Growth Horm IGF Res. 2008;18(5):369–78.
Bacon MC, White PH, Raiten DJ, Craft N, Margolis S, Levander OA, et al. Nutritional status and growth in juvenile rheumatoid arthritis. Semin Arthritis Rheum. 1990;20(2):97–106.
Ahmed SF, Sävendahl L. Promoting growth in chronic inflammatory disease: lessons from studies of the growth plate. Horm Res. 2009;72(Suppl 1):42–7.
Gaspari S, Marcovecchio ML, Breda L, Chiarelli F. Growth in juvenile idiopathic arthritis: the role of inflammation. Clin Exp Rheumatol. 2011;29(1):104–10.
MacRae VE, Farquharson C, Ahmed SF. The restricted potential for recovery of growth plate chondrogenesis and longitudinal bone growth following exposure to pro-inflammatory cytokines. J Endocrinol. 2006;189(2):319–28.
Mårtensson K, Chrysis D, Sävendahl L. Interleukin-1beta and TNF-alpha act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J Bone Min Res. 2004;19(11):1805–12.
d’Angelo DM, Di Donato G, Breda L, Chiarelli F. Growth and puberty in children with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2021;19(1):28.
Sederquist B, Fernandez-Vojvodich P, Zaman F, Sävendahl L. Recent research on the growth plate: impact of inflammatory cytokines on longitudinal bone growth. J Mol Endocrinol. 2014;53(1):T35–44.
MacRae VE, Farquharson C, Ahmed SF. The pathophysiology of the growth plate in juvenile idiopathic arthritis. Rheumatology (Oxford). 2006;45(1):11–9.
Wang SJ, Yang YH, Lin YT, Yang CM, Chiang BL. Attained adult height in juvenile rheumatoid arthritis with or without corticosteroid treatment. Clin Rheumatol. 2002;21(5):363–8.
Kearsley-Fleet L, Hyrich KL, Davies R, Lunt M, Southwood TR. Growth in children and adolescents with juvenile idiopathic arthritis over 2 years of treatment with etanercept: results from the British Society for Paediatric and adolescent rheumatology Etanercept Cohort Study. Rheumatology (Oxford). 2015;54(7):1279–85.
Tynjälä P, Lahdenne P, Vähäsalo P, Kautiainen H, Honkanen V. Impact of anti-TNF treatment on growth in severe juvenile idiopathic arthritis. Ann Rheum Dis. 2006;65(8):1044–9.
Rosenbloom AL. Idiopathic short stature: conundrums of definition and treatment. Int J Pediatr Endocrinol. 2009;2009:470378.
Backeljauw PF, Dattani MT, Cohen P, Rosenfeld RG. Disorders of growth hormone/insulin-like growth factor secretion and action. Pediatric Endocrinology: Fourth Edition: Elsevier Inc.; 2014. pp. 291–404. e1.
Wallace CA, Ruperto N, Giannini E, Arthritis C, Alliance RR, Organization PRIT, et al. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rhuematol. 2004;31(11):2290–4.
Shih YJ, Yang YH, Lin CY, Chang CL, Chiang BL. Enthesitis-related arthritis is the most common category of juvenile idiopathic arthritis in Taiwan and presents persistent active disease. Pediatr Rheumatol Online J. 2019;17(1):58.
McErlane F, Carrasco R, Kearsley-Fleet L, Baildam EM, Wedderburn LR, Foster HE, et al. Growth patterns in early juvenile idiopathic arthritis: results from the Childhood Arthritis prospective study (CAPS). Semin Arthritis Rheum. 2018;48(1):53–60.
Barut K, Adrovic A, Sahin S, Tarcin G, Tahaoglu G, Koker O, et al. Prognosis, complications and treatment response in systemic juvenile idiopathic arthritis patients: a single-center experience. Int J Rheum Dis. 2019;22(9):1661–9.
Lee JJY, Schneider R. Systemic juvenile idiopathic arthritis. Pediatr Clin North Am. 2018;65(4):691–709.
Giannini EH, Ilowite NT, Lovell DJ, Wallace CA, Rabinovich CE, Reiff A, et al. Effects of long-term etanercept treatment on growth in children with selected categories of juvenile idiopathic arthritis. Arthritis Rheum. 2010;62(11):3259–64.
Goodman SM, Springer B, Guyatt G, Abdel MP, Dasa V, George M, et al. 2017 American College of Rheumatology/American Association of Hip and knee surgeons Guideline for the Perioperative Management of Antirheumatic Medication in patients with rheumatic diseases undergoing Elective Total hip or total knee arthroplasty. Arthritis Care Res (Hoboken). 2017;69(8):1111–24.
Abdel MP, Figgie MP. Surgical management of the juvenile idiopathic arthritis patient with multiple joint involvement. Orthop Clin North Am. 2014;45(4):435–42.
Acknowledgements
We thank the Department of Statistics of National Taiwan University for the statistical analysis in this study.
Funding
The author(s) denied any funding support.
Author information
Authors and Affiliations
Contributions
HYC, YCH, YHY, BLC designed this study; HYC and YCH wrote the manuscript. YCH performed the statistical analysis. All authors were involved in clinical care and the recording of data via the Rheumatology database. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional Review Board of the National Taiwan University Hospital and was conducted according to the Declaration of Helsinki. This article was retrospectively registered due to its nature of cohort study. The informed consents from the patients were not necessary.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, HY., Hu, YC., Yang, YH. et al. Identifying factors associated with substantially reduced adult height in patients with juvenile idiopathic arthritis: a retrospective cohort study. BMC Pediatr 24, 375 (2024). https://doi.org/10.1186/s12887-024-04855-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04855-3