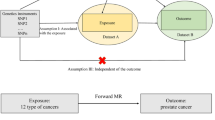
Abstract
Background
Although it is thought that prostatitis or benign prostatic hyperplasia (BPH) is related to prostate cancer (PCa), the underlying causal effects of these diseases are unclear.
Methods
We assessed the causal relationship between prostatitis or BPH and PCa using a two-sample Mendelian randomization (MR) approach. The data utilized in this study were sourced from genome-wide association study. The association of genetic variants from cohorts of prostatitis or BPH and PCa patients was determined using inverse-variance weighted and MR Egger regression techniques. The direction of chance was determined using independent genetic variants with genome-wide significance (P < 5 × 10–6). The accuracy of the results was confirmed using sensitivity analyses.
Results
MR analysis showed that BPH had a significant causal effect on PCa (Odds Ratio = 1.209, 95% Confidence Interval: 0.098–0.281, P = 5.079 × 10− 5) while prostatitis had no significant causal effect on PCa (P > 0.05). Additionally, the pleiotropic test and leave-one-out analysis showed the two-sample MR analyses were valid and reliable.
Conclusions
This MR study supports that BPH has a positive causal effect on PCa, while genetically predicted prostatitis has no causal effect on PCa. Nonetheless, further studies should explore the underlying biochemical mechanism and potential therapeutic targets for the prevention of these diseases.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is a common malignancy in men [1]. The incidence and mortality rates of PCa have increased in recent years due to the change in dietary structure and medical treatment [1]. PCa accounted for 7.3% of new male cancer cases worldwide in 2020, according to the Global Cancer Statistics from the United States [2, 3]. Besides family history/hereditary PCa [4, 5] and germline mutations [2], various exogenous and environmental factors, such as metabolic syndrome [6, 7], obesity [8, 9], and dietary factors, such as alcohol [10, 11], coffee [12], dairy [13], fat [14, 15], meat [16, 17], and vitamin D may cause PCa [18, 19].
Prostatitis and benign prostatic hyperplasia (BPH) are common prostatic diseases in men. Inflammation is closely related to the occurrence and progression of tumors [20, 21]. A retrospective study based on 746 176 participants showed that prostatitis is associated with an increased incidence rate of PCa. Furthermore, the study showed that the risk of PCa is higher in acute prostatitis than in chronic prostatitis [22]. Although many studies have assessed the relationship between BPH and the risk of PCa occurrence or death, this relationship is still unclear [23]. For instance, some studies have suggested that BPH increases [24, 25] or decreases [26] the risk of PCa, while others have found no association [27, 28]. However, these studies are mainly observational studies, which are more likely to be influenced by confounding factors.
Mendelian randomization (MR) is an epidemiological study design which limits the bias caused by common confounding and reverses causal relationships in observational studies. Genetic variants, robustly associated with a modifiable exposure, are used as instrumental variables (IVs) in MR to infer the causal relationship between the exposure and an outcome of interest [29, 30]. Two-sample MR has been widely used in various diseases due to the application of genome-wide association study (GWAS). This study aimed to evaluate the causal relationship between prostatitis or BPH and PCa based on the aggregated statistical data of large-scale GWAS using the two-sample MR analysis.
Methods
Ethical approval was not needed since all data used had been previously published in the public database. Single nucleotide polymorphisms (SNPs) were used as IVs to investigate the causal relationship between prostatitis or BPH and prostate cancer. However, this approach requires the following three assumptions: (1) SNPs must have a strong association with prostatitis and BPH; (2) SNPs should not be affected by confounders that may impact the relationship between exposure and outcome; (3) SNPs should only impact the outcome through the exposure, and not through any other pathways.
To obtain a more reliable conclusion of the causal relationship, the largest public GWAS were searched for eligible summary-level data for each trait (Table 1). Specifically, summary statistics for prostatitis, BPH, and PCa were obtained from the Integrative Epidemiology Unit (IEU) Open GWAS project. In the original literature, diagnostic criteria and inclusion procedures are listed. Additionally, the participants were of European ancestry.
The causal relationship between prostatitis or BPH and PCa was assessed using genetic instruments obtained from the MR-base database [31]. The SNPs associated with prostatitis and BPH were extracted at genome-wide significance (p < 5 × 10–6) using the stringent pairwise linkage disequilibrium (LD) r2 < 0.001 from a published GWAS meta-analysis. To make sure they were not related to any confounding factors (independence assumption), these SNPs were then checked in the database of human genotype–phenotype associations (http://www.phenoscanner.medschl.cam.ac.uk/). In addition, the IVs were evaluated based on R2 values and F statistic values to assess their correlation with exposure [32], as shown below, with the relevant variables noted.:
Note MAF, minor allele frequency; β, effect size; SE, standard error; N, sample size; k, number of SNPs.
Statistical analyses
Several MR approaches (inverse variance weighted [IVW], weighted median, and MR-Egger) were used to determine MR estimates of prostatitis for PCa and BPH for PCa after harmonization of the effect alleles across the GWASs of prostatitis or BPH and PCa. Due to the different assumptions underlying horizontal pleiotropy, multiple approaches were employed. IVW meta-analysis of the wald ratio for individual SNPs was used as the main outcome. This IVW meta-analysis assumes that instruments can affect the outcome only through the exposure of interest and not by any alternative pathway [33]. MR-Egger and weighted median methods were used to complement IVW estimates since these approaches can provide more robust estimates in a broader set of scenarios but are less efficient (wider confidence intervals [CIs]) [34].
The heterogeneity for MR estimates can be severely violated even though sensitivity analysis is crucial to detect underlying pleiotropy in MR studies. In this study, heterogeneity markers (Cochran’s Q test P < 0.05) from the IVW approach indicated potential horizontal pleiotropy. The intercept obtained from the MR-Egger regression were used to represent directional pleiotropy (P < 0.05) [35]. Additionally, horizontal pleiotropy was assessed and corrected using MR-Pleiotropy Residual Sum and Outlier methods (MR-PRESSO) [34]. Three procedures are included in MR-PRESSO: (a) detection of horizontal pleiotropy; (b) correction for horizontal pleiotropy via outlier removal; (c) testing of significant differences in the causal estimates before and after correction for outliers. When the proportion of horizontal pleiotropy variants is less than 10%, MR-PRESSO exhibits lower bias and higher precision compared to IVW and MR-Egger [36]. To determine whether a single SNP was driving or biased the MR estimate, a leave-one-out analysis was also performed. Package Two Sample MR (version 0.4.25) and MR-PRESSO (version 1.0) in R (version 3.6.1) were used for analyses.
Results
Eligible SNPs were selected as IVs to fit the three key assumptions after LD clum** (p < 5 × 10–6, LD r2 < 0.001), proxy SNP exploration, Phenoscanner database mining, and data harmonization. A total of 23 and 10 SNPs were for BPH and prostatitis, respectively. The F-statistics of more than the conventional value of 10 (F = 21.058 ~ 79.461) indicated a strong potential for these instruments, presenting a small possibility of weak instrumental variable bias. In Tables 2 and 3, we provide detailed information about IV treatments for BPH and prostatitis.
Causal effects of BPH on PCa
There was significant heterogeneity in the Cochran’s Q test (P = 0.033), and thus the IVW method was applied with a random-effect model. BPH had a significant causal effect on PCa in IVW analysis (Odds Ratio [OR] = 1.209, 95% CI: 0.098–0.281, P = 5.079 × 10− 5), similar to MR-Egger results (Fig. 1). The black line segment in the figure represents the confidence intervals. The IVW and MR-Egger MR results are presented at the bottom of the figure. However, MR-Egger regression method did not detect any directional pleiotropy (intercept = -0.0004, P = 0.196). Although MR-PRESSO analysis did not reveal any outliers, heterogeneity existed (P = 0.033). The scatter plot and funnel plot were shown in Supplementary Figure S1-2. The slope of the straight line indicates the magnitude of the causal association. Leave-one-out sensitivity analysis results are shown in Supplementary Figure S3. The black line represents the deviation of the 95% CI corresponding to the estimate of the SNPs. The red line represents the estimated value of the IVW test. There was no difference with the final result after the removal of SNPs one by one.
Causal effects of prostatitis on PCa
The fixed-effect IVW models were used in the main analysis after Cochran’s Q test due to the lack of heterogeneity. MR analysis demonstrated that genetically predicted prostatitis was not associated with PCa (OR = 1.001, 95% CI: -0.0002-0.002, P = 0.12, Fig. 2). The MR-Egger regression method did not identify horizontal pleiotropy (intercept = 0.0003, P = 0.79). Although MR-PRESSO analysis did not reveal any outliers, heterogeneity existed (P = 0.081). The scatter plot and funnel plot are shown in Supplementary Figure S4-5, while leave-one-out sensitivity analysis is shown in Supplementary Figure S6.
Discussion
This study aimed to identify a potential causal relationship between prostatitis or BPH and PCa based on a two-sample MR approach, providing directions for further mechanistic investigations.
Some epidemiological studies have in recent years shown that BPH is associated with PCa, consistent with this study. Katharina Boehm et al. found that a BPH is associated with an increased risk of PCa based on a population-based case-control study (PROtEuS), especially for low-grade PCa [37]. Furthermore, a 27-year follow-up cohort study with 3 009 258 Danish men found that clinical BPH is associated with a two- to three-fold increased risk of PCa incidence and a two- to eight-fold increased risk of PCa mortality [25]. Although these studies matched age and ethnicity to minimize confounding effects, the inherent limitations of retrospective studies cannot be avoided.
In this study, results showed that BPH is a risk factor for PCa from a genetic perspective. Previous studies identified several potential mechanisms by which BPH exerts this effect. The common pathogenesis of BPH and PCa is age, genetics [38, 39], and the common pathogenesis of both, such as androgens, inflammation, obesity, metabolic syndrome, and diet [40,41,42,43,44,45]. Moreover, the process of seeking medical treatment for BPH often involves screening for PCa.
Although previous biological and epidemiological studies have shown that prostatitis is a risk factor for prostate cancer, the lack of cohort studies makes it difficult to conclude that there is a causal relationship between prostatitis and PCa. In this research, results showed that prostatitis did not influence the overall risk of PCa.
Inflammation, especially chronic inflammatory conditions, is associated with cancer development and progression. For instance, reflux esophagitis and virus hepatitis are associated with oesophageal cancer and hepatocellular carcinoma, respectively. Chronic inflammation induces carcinogenesis due to the local irritation associated with the regulation of the inflammatory cells and cytokine [46]. Both bacteria- and non-bacteria-related prostatitis (except for sexually transmitted diseases) are significantly associated with prostate cancer [47, 48]. Gyoohwan Jung et al. found that the incidence of PCa is significantly increased in 746 176 prostatitis patients (Hazard Ratio [HR] 2.99; 95% CI 2.89–3.09, p < 0.001). In that study, the HR for PCa was significantly higher in acute prostatitis than in chronic prostatitis (3.82 vs. 2.77) [22]. In an analysis of 167 autopsied prostates, Delongchamps et al. concluded chronic inflammation is frequently associated with BPH, but not with cancer [49]. Furthermore, chronic inflammatory infiltrations are located in the transitional zones instead of the peripheral zone where prostate cancer is usually diagnosed [50, 51]. However, the causality remains unclear.
However, it is difficult to confirm the causal relationship between prostatitis and PCa solely based on observational research since correlation studies cannot answer the question of causality. In summary, these findings should be carefully interpreted. Unlike most observational studies, we did not find a causal relationship between prostatitis and PCa, possibly due to use of different analytical methods.
This MR research has several advantages. First, to the best of our knowledge, this is the first study to evaluate the causal relationship between BPH and prostatitis on PCa using a dual sample MR analysis based on large-scale GWAS data. Compared with previous observational studies, MR analysis can effectively reduce potential biases, including confounding factors and reverse causal relationships, thereby enhancing causal inference. Second, the GWAS dataset for prostatitis, BPH, and PCa used is mainly based on populations of European ancestry and thus can minimize the impact of population stratification. Third, different estimation models and strict sensitivity analysis were used to ensure the reliability of the results.However, this study has some limitations. First, the study results do not represent a truly random population sample and are not applicable to other races since the data represent populations of European ancestry. Second, there may be some overlap in exposure and outcomes among participants, which can reduce data quality. Third, although various sensitivity analyses have been conducted to test the hypotheses of MR studies, it is also difficult to completely rule out the level pleiotropy of IVs. Pleiotropy broadly refers to SNPs being associated with effects in more than one trait. We can’t thoroughly rule out pleiotropic effects of the SNPs included in prostatitis or BPH that may confound the association, though we carefully selected the SNPs to avoid that. Finally, the current sample size of GWAS data is still large enough, and thus more GWAS data are needed to verify these findings.
Conclusion
This MR study supports that BPH has a positive causal effect on PCa, while genetically predicted prostatitis has no causal effect on prostatitis. Nonetheless, more studies should explore the underlying biochemical mechanism and potential therapeutic targets for the prevention of BPH, prostatitis, and PCa.
Data availability
All data used in the current study are publicly available GWAS summary data (https://gwas.mrcieu.ac.uk/).
Abbreviations
- A:
-
Adenine
- BETA:
-
Effect size
- BPH:
-
Benign prostatic hyperplasia
- C:
-
Cytosine
- CI:
-
Confidence interval
- EA:
-
Effect allele
- EAF:
-
Effect allele frequency
- G:
-
Guanine
- GWAS:
-
Genome-wide association study
- HR:
-
Hazard Ratio
- IEU:
-
Integrative Epidemiology Unit
- IV:
-
Instrumental variable
- IVW:
-
Inverse variance weighted
- LD:
-
Linkage disequilibrium
- MAF:
-
Minor allele frequency
- MRC:
-
Medical Research Council
- MR:
-
Mendelian randomization
- NEA:
-
Other allele
- OR:
-
Odds Ratio
- PCa:
-
Prostate cancer
- SE:
-
Standard error
- SNP:
-
Single nucleotide polymorphism
- T:
-
Thymine
References
Kimura T, Sato S, Takahashi H, Egawa S. Global trends of latent prostate Cancer in autopsy studies. Cancers (Basel). 2021;13(2).
Giri VN, Hegarty SE, Hyatt C, et al. Germline genetic testing for inherited prostate cancer in practice: implications for genetic testing, precision therapy, and cascade testing. Prostate. 2019;79(4):333–9.
Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Hemminki K. Familial risk and familial survival in prostate cancer. World J Urol. 2012;30(2):143–8.
Jansson KF, Akre O, Garmo H, et al. Concordance of tumor differentiation among brothers with prostate cancer. Eur Urol. 2012;62(4):656–61.
Blanc-Lapierre A, Spence A, Karakiewicz PI, Aprikian A, Saad F, Parent MÉ. Metabolic syndrome and prostate cancer risk in a population-based case-control study in Montreal, Canada. BMC Public Health. 2015;15:913.
Esposito K, Chiodini P, Capuano A, et al. Effect of metabolic syndrome and its components on prostate cancer risk: meta-analysis. J Endocrinol Invest. 2013;36(2):132–9.
Rivera-Izquierdo M, Pérez de Rojas J, Martínez-Ruiz V et al. Obesity as a risk factor for prostate Cancer mortality: a systematic review and dose-response Meta-analysis of 280,199 patients. Cancers (Basel). 2021;13(16).
Vidal AC, Howard LE, Moreira DM, Castro-Santamaria R, Andriole GL Jr, Freedland SJ. Obesity increases the risk for high-grade prostate cancer: results from the REDUCE study. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2936–42.
Dickerman BA, Markt SC, Koskenvuo M, Pukkala E, Mucci LA, Kaprio J. Alcohol intake, drinking patterns, and prostate cancer risk and mortality: a 30-year prospective cohort study of Finnish twins. Cancer Causes Control. 2016;27(9):1049–58.
Zhao J, Stockwell T, Roemer A, Chikritzhs T. Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta-analysis. BMC Cancer. 2016;16(1):845.
Chen X, Zhao Y, Tao Z, Wang K. Coffee consumption and risk of prostate cancer: a systematic review and meta-analysis. BMJ Open. 2021;11(2):e038902.
Key TJ. Nutrition, hormones and prostate cancer risk: results from the European prospective investigation into cancer and nutrition. Recent Results Cancer Res. 2014;202:39–46.
Alexander DD, Bassett JK, Weed DL, Barrett EC, Watson H, Harris W. Meta-analysis of long-chain Omega-3 polyunsaturated fatty acids (LCω-3PUFA) and prostate Cancer. Nutr Cancer. 2015;67(4):543–54.
Lippi G, Mattiuzzi C. Fried food and prostate cancer risk: systematic review and meta-analysis. Int J Food Sci Nutr. 2015;66(5):587–9.
Bylsma LC, Alexander DD. A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer. Nutr J. 2015;14:125.
Nouri-Majd S, Salari-Moghaddam A, Aminianfar A, Larijani B, Esmaillzadeh A. Association between Red and processed meat consumption and risk of prostate Cancer: a systematic review and Meta-analysis. Front Nutr. 2022;9:801722.
Kristal AR, Till C, Song X, et al. Plasma vitamin D and prostate cancer risk: results from the selenium and vitamin E Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1494–504.
Nyame YA, Murphy AB, Bowen DK, et al. Associations between serum vitamin D and adverse Pathology in men undergoing radical prostatectomy. J Clin Oncol. 2016;34(12):1345–9.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Jung G, Kim JK, Kim H, Lee J, Hong SK. The association between prostatitis and risk of prostate cancer: a National Health Insurance Database study. World J Urol. 2022;40(11):2781–7.
Alcaraz A, Hammerer P, Tubaro A, Schröder FH, Castro R. Is there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature review. Eur Urol. 2009;55(4):864–73.
Chokkalingam AP, Nyrén O, Johansson JE, et al. Prostate carcinoma risk subsequent to diagnosis of benign prostatic hyperplasia: a population-based cohort study in Sweden. Cancer. 2003;98(8):1727–34.
Ørsted DD, Bojesen SE, Nielsen SF, Nordestgaard BG. Association of clinical benign prostate hyperplasia with prostate cancer incidence and mortality revisited: a nationwide cohort study of 3,009,258 men. Eur Urol. 2011;60(4):691–8.
Freedland SJ, Isaacs WB, Platz EA, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005;23(30):7546–54.
Schenk JM, Kristal AR, Arnold KB, et al. Association of symptomatic benign prostatic hyperplasia and prostate cancer: results from the prostate cancer prevention trial. Am J Epidemiol. 2011;173(12):1419–28.
Simons BD, Morrison AS, Young RH, Verhoek-Oftedahl W. The relation of surgery for prostatic hypertrophy to carcinoma of the prostate. Am J Epidemiol. 1993;138(5):294–300.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–98.
Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an Approach to assess causality using Observational Data. J Am Soc Nephrol. 2016;27(11):3253–65.
Hemani G, Zheng J, Elsworth B et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–74.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Ong JS, MacGregor S. Implementing MR-PRESSO and GCTA-GSMR for pleiotropy assessment in mendelian randomization studies from a practitioner’s perspective. Genet Epidemiol. 2019;43(6):609–16.
Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Boehm K, Valdivieso R, Meskawi M, et al. BPH: a tell-tale sign of prostate cancer? Results from the prostate Cancer and Environment Study (PROtEuS). World J Urol. 2015;33(12):2063–9.
Parsons JK. Benign Prostatic Hyperplasia and male lower urinary tract symptoms: epidemiology and risk factors. Curr Bladder Dysfunct Rep. 2010;5(4):212–8.
Pearson JD, Lei HH, Beaty TH, et al. Familial aggregation of bothersome benign prostatic hyperplasia symptoms. Urology. 2003;61(4):781–5.
Di Silverio F, Gentile V, De Matteis A, et al. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43(2):164–75.
Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation. 2003;108(13):1541–5.
Kristal AR, Arnold KB, Schenk JM, et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2008;167(8):925–34.
Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84(9):976–81.
Parsons JK, Carter HB, Partin AW, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91(7):2562–8.
Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol. 2009;182(6 Suppl):S27–31.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7.
Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60(1):78–83.
Roberts RO, Bergstralh EJ, Bass SE, Lieber MM, Jacobsen SJ. Prostatitis as a risk factor for prostate cancer. Epidemiology. 2004;15(1):93–9.
Delongchamps NB, de la Roza G, Chandan V, et al. Evaluation of prostatitis in autopsied prostates–is chronic inflammation more associated with benign prostatic hyperplasia or cancer. J Urol. 2008;179(5):1736–40.
McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12(12):897–906.
Pavelić J, Zeljko Z. [Prostate gland-transition zone lesions. Etiology, growth regulation, growth factors, genetic changes]. Lijec Vjesn. 2002;124(6–7):211–9.
Acknowledgements
Thanks for the contribution of the participantsand Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Funding
This work was supported by the Foundation of **aoshan Science and Technology Bureau of Hangzhou, China [grant number 2020210]; and Zhejiang Provincial Natural Science Foundation of China [grant number LQ21H050003].
Author information
Authors and Affiliations
Contributions
JH, YF and BQ conceived and designed the study. JH, JS, KW and LZ contributed to data curation. JH and JS contributed to methodology and visualization. JH wrote the original manuscript. YF and BQ revised the article and contributed to the final version of the manuscript. All authors have reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval was not needed since all data used had been previously published in the public database.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, J., Sun, J., Wang, K. et al. Causal relationship between prostatic diseases and prostate cancer: a mendelian randomization study. BMC Cancer 24, 774 (2024). https://doi.org/10.1186/s12885-024-12551-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12551-9