Abstract
Background
Natural Killer (NK) cells play an important role in tumor prevention, but once tumors form, the numbers as well as the cytotoxic functions of NK cells are reduced. IL-15 is a cytokine that increases and activates NK cells. Here we will examine the anti-tumor role of IL-15 in a spontaneous breast cancer model.
Methods
To achieve this, Polyoma Middle T (MT) mice that form spontaneous breast cancer were crossed with mice that either overexpress IL-15 (IL-15 transgenic (TG)) or mice that lack IL-15 (IL-15 knockout (KO)). We compared survival curves and tumor formation in IL-15 KO/MT, MT and IL-15 TG/MT groups. In addition, the phenotype, activation and contribution of NK cells and CD8 T cells to tumor formation were examined in each of these mouse strains via flow cytometry, ELISA, adoptive transfer and antibody depletion experiments.
Results
IL-15KO/MT tumors formed and progressed to endpoint more quickly than MT tumors. These tumors displayed little apoptosis and poor CD8 T cell infiltration. In contrast, IL-15 TG/MT mice had increased survival and the tumors displayed extensive cell death, high proportions of activated NK cells and a higher infiltration of CD8 T cells than MT tumors. CD8 T cells in IL-15 TG/MT tumors were capable of secreting IFNγ, possessed markers of memory, did not display an exhausted phenotype and were frequently NK1.1+. Long-term antibody depletion studies in IL-15 TG/MT mice revealed that NK1.1+, but not CD8 T cells, were critical for tumor destruction. Lastly, human NK cells, when exposed to a similar cytokine environment as that found in IL-15TG/MT tumors, were capable of killing human breast cancer cells.
Conclusions
This study reveals that high levels of IL-15 can promote tumor destruction and reduce metastasis in breast cancer via effects on NK1.1+ cells. Our results suggest that strategies aimed at increasing NK cell activation may be effective against solid epithelial cancers.
Similar content being viewed by others
Background
Natural Killer (NK) cells were discovered almost 40 years ago due to their ability to kill tumor cells with no prior sensitization [1]. Since then, extensive knowledge has been gained about their instrumental role in tumor immunosurveillance [2]. NK cells are capable of killing tumor cells via multiple mechanisms. The ability of an NK cell to kill another cell is controlled by a balance of activating and inhibitory receptors expressed on their cell surface that allow the NK cells to sense self versus damaged cells [3]. Recently, it has been shown that in addition to preventing tumor formation, NK cells can eradicate large solid tumors [4] and kill mammary cancer stem cells [5]. Unfortunately, in several malignancies, including breast cancer, NK cell activity as well as expression of activating receptors, is often suppressed [6,7]. Tumors promote this down regulation by the secretion of molecules such as TGF β and IL-10 [7-9]. Recent reports suggest that NK cells within the tumor may actually support tumor growth [10,11]. Alterations in NK cell activity are reversible, as NK cells rapidly respond to their environment [7,12]. The ability to shift the NK cell phenotype from inhibition/tumor promotion to activation will be essential for the use of NK cells against cancer.
IL-15 is a cytokine that has effects on both the innate and the adaptive immune system. IL-15 promotes the differentiation, proliferation and activation of NK cells and the formation of a subset of memory CD8 T cells [13-15]. This has been confirmed in IL-15 TG mice which have increased activated NK cells and increased proportions of memory CD8 T cells, whereas IL-15 KO mice lack NK cells and have decreased CD8 T cells [16,17]. The ability of IL-15 to promote both NK cell and CD8 T cell responses has led to interest in IL-15 as a cancer immunotherapy. Most in vivo studies investigating the effects of IL-15 have used subcutaneous engrafted or lung metastasis cancer models. For example, several studies found that IL-15 TG mice were resistant to engrafted tumor formation [18,19]. IL-15 has been administered by several routes and use of each of these methods has impaired tumor growth or metastasis [20-25]. The protection observed was either NK cell and/or CD8 T cell dependent [18-20,22]. While many treatment strategies have been successful in engrafted and metastatic models, it is unknown if this will translate into a spontaneous epithelial cancer model where tumors initiate and grow alongside an intact tolerized immune system.
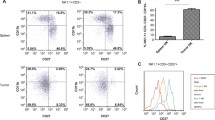
In this study, we crossed IL-15 KO and IL-15 TG mice with a spontaneous breast cancer model (MT) to create IL-15 KO/MT and IL-15 TG/MT mice. MT mice express the polyoma MT antigen under the mouse mammary tumor virus long terminal repeat [26]. In MT mice, multifocal adenocarcinomas form and these frequently metastasize to the lung [26]. The MT model on a C57BL/6 background is a good model of human breast cancer as tumor formation is sequential and goes from focal hyperplasia to mammary intraepithelial neoplasms to carcinoma in situ and ends with multiple invasive tumors [27,1: Figure S2A). These NK1.1 + CD8 T cells were absent in IL-15 KO/MT tumors and much lower in MT tumors (Additional file 1: Figure S2A). To determine if these cells produced high levels of IFNγ, we isolated NK1.1+ cells from IL-15 TG/MT tumors and performed non-specific stimulation (CD3/28) in the presence of protein secretion inhibitors. 11-13% of CD3 + CD8+ NK1.1+ T cells produced IFNγ, a similar percent to that seen for total CD8 T cells that produced IFNγ (Additional file 1: Figure S2B, 5G). Therefore, in our model, these cells were capable of producing IFNγ, but were not the only CD8 T cells doing so.
Cells expressing NK1.1 are responsible for tumor destruction in IL-15 TG/MT mice
To determine which cell type(s) were responsible for anti-tumor responses in IL-15 TG/MT mice, we performed long term antibody depletion experiments with anti-NK1.1 or anti-CD8α antibody in IL-15 TG/MT mice. NK1.1 depletion efficiently depleted NK1.1+ cells within the spleen and tumor (Additional file 1: Figure S3A). IL-15 TG/MT mice that were depleted of NK1.1+ cells formed tumors faster and proceeded to endpoint more quickly than IL-15 TG/MT mice (p < 0.001, 0.0001, respectively) (Figure 6A). In fact, tumor formation closely followed what was observed in IL-15 KO/MT mice. In addition, histological analysis revealed that the tumor destruction in IL-15 TG/MT mice was absent when these NK1.1+ cells were absent (Figure 6C). The anti-CD8α antibody removed CD8 expressing cells from the spleen of IL-15 TG/MT mice, but only partially from the tumor (at least by 2/3 ie. 18% to 6%)(Additional file 1: Figure S3B). The majority of the CD3 + CD8+ T cells that were left in the depleted tumor were NK1.1+ (Additional file 1: Figure S3C). There were no statistically significant differences between the IL-15 TG/MT and the IL-15 TG/MT CD8 depleted mice (Figure 6B). Lastly, the tumors that formed in IL-15 TG/MT CD8 depleted mice had similar tumor destruction to that seen in normal IL-15 TG/MT mice (Figure 6C). Therefore, NK1.1+ cells play a major role in the tumor destruction and extension of survival in IL-15 TG/MT mice, whereas CD8 T cells, although activated phenotypically, play less of a role.
The effect of NK1.1 and CD8α depletion on tumor formation. (A-B) IL-15 TG/MT mice were depleted with anti-NK1.1 (n = 6) (A) or anti-CD8α (n = 5) (B) antibody long term starting at 4 weeks of age. (A) In comparison to the tumor formation and survival curves for IL-15 TG/MT mice (n = 36 for percent tumor free, n = 28 for survival), NK1.1 depleted IL-15 TG/MT mouse tumors formed and progressed to endpoint more quickly. IL-15 TG/MT NK1.1 depleted mice were not different from IL-15 KO/MT tumor mice (n = 30). (B) In comparison to the tumor formation and survival curves for IL-15 TG/MT mice, CD8 depleted IL-15 TG/MT mouse tumors formed and progressed to endpoint at a similar rate. (C) Tumors that formed in IL-15 TG/MT NK1.1 depleted mice did not show the extensive destruction seen in normal IL-15 TG/MT tumors and in IL-15 TG/MT CD8 depleted mice. Arrows indicate areas of tumor destruction. **p < 0.01, ***p < 0.001.
Adoptive transfer of CD8 T cells from IL-15 TG/MT mice does not lead to protection from tumor challenge
To further examine the impact of CD8 T cells from IL-15 TG/MT mice on tumor formation, we performed CD8 T cell adoptive transfers. C57BL/6 recipient mice were treated with cyclophosphamide to induce lymphopenia. 24 hours later CD8 T cells were isolated from the spleens/tumors of MT, IL-15 TG/MT and IL-15 TG mice, labelled with CFSE (5 × 106 spleen, 1 × 106 tumor) and injected IV. 24 hours after transfer, mice were challenged with a sub-cutaneous dose of fresh primary MT tumors from which immune cells had been removed. Mice were followed for tumor formation and endpoint. 8 days after challenge, several mice were sacrificed to ensure that the adoptive transfer was successful (4-8% of total CD8 T cells were CFSE positive - data not shown). There were no statistically significant differences in tumor formation or endpoint between control mice that received no CD8 T cells and mice that received CD8 T cells from MT, IL-15 TG/MT or IL-15 TG spleens or IL-15 TG/MT tumors (Additional file 1: Figure S4).
The effect of cytokines on the ability of human NK cells to kill a human breast cancer cell line
IL-15 overexpression in MT breast tumors created an environment where several cytokines that affect NK cell activation and proliferation were altered. We examined the expression of these cytokines in IL-15 TG/MT tumors versus MT or IL-15 KO/MT tumors and found that in addition to IL-15, both IL-12 and IL-18 were increased within IL-15 TG/MT tumors (Figure 7A/B). To determine if exposure to these 3 cytokines simultaneously would affect the ability of NK cells to kill breast tumor cells, we isolated NK cells from human PBMCs and stimulated them in an IL-15/IL-12/IL-18 rich environment for 16 hours. We then co-incubated them with a CFSE labelled MDA-231 human breast cancer cell line at various E:T ratios for 5 hours to determine their killing potential. The gating strategy used can be seen in Figure 7C and included exclusion of CD45+ NK cells, selection of CFSE+ tumor cells and finally the percent of CFSE+ tumor cells that were dead (7AAD+). MT cells alone were included as the level of basal MT cell death. In contrast to NK cells cultured in IL-2 alone, NK cells cultured in IL-15/IL-12/IL-18 were highly cytotoxic toward this breast cancer cell line and at E:T ratios of 10:1 reached specific lysis levels of 52% (Figure 7C/D).
Cytokines in IL-15 TG/MT tumors and their effects on the ability of human NK cells to kill human breast cancer cells. (A & B) IL-12 and IL-18 are increased in IL-15 TG/MT tumors. (A) IL-12 ELISAs were performed on tumor homogenates from 6 size matched tumors per group. (B) 3 tumors from different mice in each tumor group were pooled (size matched between groups) and assayed for IL-18 cytokine levels via multianalyte protein analysis. (C & D) NK cells isolated from human PBMCs were cultured in IL-2 or IL-15/IL-12/IL-18 for 16 hours before being incubated with CFSE labelled MDA-231 cells for 5 hours in a killing assay. In the demonstration of the gating strategy in C) CD45+ NK cells were first removed from the analysis and then CFSE+ MT cells were selected. 7AAD was used to determine the percentage of the CFSE+ MT cells that were dead. Results at an E:T ratio of 1:10 are displayed (E:T = NKcell:MDA-231). MT alone is included to show spontaneous death levels which were used in the calculation of D. (D) Percent specific lysis of MDA-231 in the killing assay at various E:T ratios. Representative of 2 experiments. *p < 0.05.
Discussion
While IL-15 has been under investigation as a cancer immunotherapeutic for the last decade, investigation has focused on tumor models that do not closely mimic spontaneous tumor formation in humans. It is known that injecting tumor cell lines subcutaneously or intravenously is a useful, but rather artificial system in which it is easier to develop immune responses to the tumor. In addition, there is a lack of studies that have examined the impact of IL-15 on solid epithelial tumors such as breast cancer. To examine the role of IL-15 in a more relevant model we utilized an immunologically tolerant mouse model of spontaneous mammary tumor formation (MT) and examined tumor formation in the absence of IL-15 (IL-15 KO/MT) or with IL-15 overexpression (IL-15 TG/MT). Overall, IL-15 TG/MT mice had increased survival when compared to either MT or IL-15 KO/MT mice. In contrast IL-15 KO/MT mice had faster tumor formation and decreased survival when compared to MT or IL-15 TG/MT mice. These results are similar to the anti-tumor effects of IL-15 that have been observed in other engrafted and metastatic models of melanoma and colon cancer, but it is one of the first reports of this in a spontaneous model of breast cancer [18,19,21].
IL-15TG/MT mice formed tumors, but these tumors had a very different phenotype than IL-15 KO/MT or MT tumors. This included large areas of cell death, a higher proportion of NK cells as well as increased CD8 T cell infiltration. Increased CD8 T cells or NK cells within the tumor is a positive prognostic factor in many human and mouse tumor types [32,36,37]. In many cases though, NK cells within the tumor express inhibitory receptors instead of activating receptors and have low cytotoxicity [7,38]. To examine NK cell phenotype, we identified NK cells using flow cytometry as NK1.1 + CD3- cells. NK1.1 is commonly used as a marker for NK cells in C57BL/6 mice. It is a member of the NKRP1 receptor family and while it is thought to be an activating receptor its’ ligand is unknown [39-41]. The NK cells within IL-15 TG/MT tumors possessed higher levels of both activating receptors (NKG2D, NKp46) and other markers of activation (CD69). Recently, ligands for NKG2D and NKp46 were found to be expressed on human primary breast tumors and breast tumor cell lines [9]. In addition, blockade of either NKp46 or NKG2D decreased the ability of NK cells to kill breast tumor cells that expressed ligands to these receptors [9]. We also found that there was a higher percentage of CD27high NK cells in IL-15 TG/MT tumors than in MT tumors. CD27 expression, in addition to CD11b expression, has been used to define mature mouse NK cells into subsets [30,42]. In progression from less mature to more mature: CD27lowCD11blow to CD27highCD11blow to CD27highCD11bhigh to CD27lowCD11bhigh [42]. Importantly, CD27high NK cells were found to have a higher degree of effector function, including cytotoxicity and cytokine production [30]. More recently it was found that CD27 on human NK cells could also be used to define NK cell subsets [43,44]. In contrast to what has been found with mouse NK cells, human CD27+ NK cells have been associated with low cytotoxic activity and high ability to secrete cytokines [43,44]. Overall, NK cells found within IL-15 TG/MT tumors are likely more capable of killing breast tumor cells than those found in MT tumors.
We observed increased CD8 T cells within IL-15 TG/MT tumors, but CD8 T cells within the tumor are not always functional as they can be anergic and/or exhausted [33,35]. While IL-15 KO/MT tumor CD8 T cells had high levels of exhaustion markers (PD-1) and lacked IFNγ production, IL-15 TG/MT CD8 T cells had very low levels of PD-1 and produced large amounts of IFNγ. This is in contrast to another report that found that treatment with IL-15 in a metastatic model of colon carcinoma led to increased PD-1 expression on CD8 T cells in the spleen [45]. It is likely that the short term administration of IL-15 or the model system used accounted for the discrepancies in our observations. In addition, a higher proportion of CD8 T cells in the IL-15 TG/MT tumors were CD44+ and CD62Lhigh, which are markers of central memory CD8 T cells. Central memory CD8 T cells are thought to be extremely effective in anti-tumor defence [31,46]. We also observed a high proportion of unique NK1.1+ CD8 T cells in IL-15 TG/MT tumors. This cell type has been previously identified as highly cytotoxic (high perforin/granzyme level) and able to produce large amounts of IFNγ [47,48]. In our model, while some of these cells produce IFNγ, they were not the major source. While we have not examined this here, it is interesting to speculate about whether these unique CD8 T cells developed in the tumor or migrated to the tumor from elsewhere. We do know that a low percentage of these cells can be found in IL-15 TG mice in other organs such as the spleen (data not shown), so they are not completely unique to the tumor environment. The ability of IL-15 to induce expression of other NK cell markers such as CD56 in human CD8 T cells has also been reported in a variety of models [49,50]. Thus, this effect does not appear to be limited to our mouse models or to one type of NK cell receptor.
In previous studies involving IL-15, protective effects were found to be NK cell or CD8 T cell dependent [18,19,22]. In IL-15 TG/MT mice, NK1.1 positive (includes NK cells, NKT cells, NK1.1+ CD8 T cells) but not CD8 positive cells were the most important cells for increased survival and tumor destruction. The CD8 depletion was substantial but not complete in the tumor and the majority of CD8 T cells that remained in the tumor were NK1.1+, indicating that this cell type was resistant to depletion via this method. It has previously been reported that these cells may be resistant to activation-induced cell death and this may contribute to the inefficient depletion [48]. Since CD3 + CD8 + NK1.1+ cells were removed by the NK1.1 depletion, we cannot rule out a role for this cell type in the tumor destruction of IL-15 TG/MT mice. The lack of contribution of CD8 T cells to increased survival was surprising due to the fact that they existed in such high numbers and were of the correct phenotype to fight cancer. To confirm this data, we performed a CD8 T cell adoptive transfer experiment. Despite the fact that CFSE labelled CD8 T cells were present in the spleen and tumor at endpoint (data not shown), there was no impact on survival from transfer of either IL-15 TG/MT, MT or IL-15 TG splenic CD8 T cells or IL-15 TG/MT tumor CD8 T cells after a subcutaneous primary MT tumor injection. It is possible that either this aggressive tumor formed too fast for the transferred CD8 T cells to have an impact or that the tumor rapidly lost MHC I expression to compensate for the presence of tumor specific CD8 T cells. Also, MT tumor formation has been found to be slightly different each time and different tumors may express different tumor antigens, some even lose expression of MT itself [51]. Thus, it is possible that despite taking MT tumor cells to inject from multiple mice and CD8 T cells from multiple mice and pooling them, they may still not have been specific for that tumor. In terms of MHC I loss, a similar phenomenon may be occurring in the spontaneous model. It is also possible that IL-15 overexpression may induce non-specific proliferation of CD8 T cells, not tumor specific responses [52]. This data indicates that overexpression of IL-15 can generate an anti-tumoral NK cell response that is effective at extending survival in the MT model.
Another promising finding revealed in this model was that overexpression of IL-15 appears to delay the formation of lung metastases. This observation, in a spontaneous model of breast tumor metastasis, strongly indicates that IL-15 has potential therapeutically to prevent metastasis. Previously, this has only been examined in injected models of metastasis. This may be very useful in a clinical setting in which metastasis is frequent and leads to significant increases in mortality.
Conclusions
IL-15 is a promising new cancer therapeutic that is well tolerated in primate models [53]. It appears to be superior to IL-2 as it has lower toxicity, does not increase T regulatory cells and induces higher levels of NK cell and CD8 T cell effector responses [53,54]. Based on the success of IL-15 in animal models, the first clinical trials have begun (NCT01727076, NCT01021059, NCT01572593) in multiple tumor types including melanoma, renal cell carcinoma and non-small cell lung carcinoma patients. Recently, there has been renewed interest in NK cells as a target to activate in the fight against breast cancer. It has been shown that human breast cancer cells express activating ligands as well as death-inducing receptors- both of which NK cells use to correctly identify their target cells [9,55]. In fact, expression of NKG2D ligands in human breast cancer was associated with a significant beneficial outcome [56]. It has also been established that NK cells are capable of eradicating a solid epithelial cancer (fibrosarcoma) and that they may also be able to target breast cancer stem-cell like cells [4,5]. Here, we found that when human NK cells were exposed to a similar cytokine environment to that found in IL-15 overexpressed MT tumors, they were highly capable of killing a triple negative breast cancer cell line. Other studies have reported that NK cells grown in IL-15, IL-12 and IL-18 are thought to display long term effector functions and may be memory-like NK cells [57,58]. These studies, along with our data, lends credence to the idea that stimulating innate immune cells such as NK cells can be effective clinically against breast cancer primary tumors and metastasis.
References
Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–7.
Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932–43.
Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10.
Liu RB, Engels B, Arina A, Schreiber K, Hyjek E, Schietinger A, et al. Densely granulated murine NK cells eradicate large solid tumors. Cancer Res. 2012;72(8):1964–74.
Li M, Knight DA, Smyth MJ, Stewart TJ. Sensitivity of a novel model of mammary cancer stem cell-like cells to TNF-related death pathways. Cancer Immunol Immunother. 2012;61(8):1255–68.
Konjevic G, Jurisic V, Jovic V, Vuletic A, Mirjacic Martinovic K, Radenkovic S, et al. Investigation of NK cell function and their modulation in different malignancies. Immunol Res. 2012;52(1-2):139–56.
Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121(9):3609–22.
Mamessier E, Bertucci F, Sabatier R, Birnbaum D, Olive D. "Stealth" tumors: Breast cancer cells shun NK-cells anti-tumor immunity. Oncoimmunology. 2012;1(3):366–8.
Mamessier E, Sylvain A, Bertucci F, Castellano R, Finetti P, Houvenaeghel G, et al. Human breast tumor cells induce self-tolerance mechanisms to avoid NKG2D-mediated and DNAM-mediated NK cell recognition. Cancer Res. 2011;71(21):6621–32.
Gillard-Bocquet M, Caer C, Cagnard N, Crozet L, Perez M, Fridman WH, et al. Lung tumor microenvironment induces specific gene expression signature in intratumoral NK cells. Front Immunol. 2013;4:19.
Bruno A, Focaccetti C, Pagani A, Imperatori AS, Spagnoletti M, Rotolo N, et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia. 2013;15(2):133–42.
Konjevic G, Spuzic I. Stage dependence of NK cell activity and its modulation by interleukin 2 in patients with breast cancer. Neoplasma. 1993;40(2):81–5.
Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101(12):4887–93.
Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100(10):3633–8.
Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196(7):935–46.
Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193(2):219–31.
Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–80.
Yajima T, Nishimura H, Wajjwalku W, Harada M, Kuwano H, Yoshikai Y. Overexpression of interleukin-15 in vivo enhances antitumor activity against MHC class I-negative and -positive malignant melanoma through augmented NK activity and cytotoxic T-cell response. Int J Cancer. 2002;99(4):573–8.
Kobayashi H, Dubois S, Sato N, Sabzevari H, Sakai Y, Waldmann TA, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105(2):721–7.
Bessard A, Sole V, Bouchaud G, Quemener A, Jacques Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol Cancer Ther. 2009;8(9):2736–45.
Ugen KE, Kutzler MA, Marrero B, Westover J, Coppola D, Weiner DB, et al. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther. 2006;13(10):969–74.
Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68(8):2972–83.
Chang CM, Lo CH, Shih YM, Chen Y, Wu PY, Tsuneyama K, et al. Treatment of Hepatocellular Carcinoma with Adeno-associated Virus Encoding Interleukin-15 Superagonist. Hum Gene Ther. 2010;21(5):611–21.
Tang F, Zhao LT, Jiang Y, de Ba N, Cui LX, He W. Activity of recombinant human interleukin-15 against tumor recurrence and metastasis in mice. Cell Mol Immunol. 2008;5(3):189–96.
Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180(4):2099–106.
Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12(3):954–61.
Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163(5):2113–26.
**a J, Tanaka Y, Koido S, Liu C, Mukherjee P, Gendler SJ, et al. Prevention of spontaneous breast carcinoma by prophylactic vaccination with dendritic/tumor fusion cells. J Immunol. 2003;170(4):1980–6.
Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176(3):1375–85.
Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176(3):1517–24.
Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(7):1969–74.
Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008;222:101–16.
Srinivasan M, Frauwirth KA. Peripheral tolerance in CD8+ T cells. Cytokine. 2009;46(2):147–59.
Wang RF. CD8+ regulatory T cells, their suppressive mechanisms, and regulation in cancer. Hum Immunol. 2008;69(11):811–4.
Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44.
Street SE, Zerafa N, Iezzi M, Westwood JA, Stagg J, Musiani P, et al. Host perforin reduces tumor number but does not increase survival in oncogene-driven mammary adenocarcinoma. Cancer Res. 2007;67(11):5454–60.
Marrogi AJ, Munshi A, Merogi AJ, Ohadike Y, El-Habashi A, Marrogi OL, et al. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer. 1997;74(5):492–501.
Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112(4):863–75.
Vogler I, Steinle A. Vis-a-vis in the NKC: genetically linked natural killer cell receptor/ligand pairs in the natural killer gene complex (NKC). J Innate Immun. 2011;3(3):227–35.
Bartel Y, Bauer B, Steinle A. Modulation of NK cell function by genetically coupled C-type lectin-like receptor/ligand pairs encoded in the human natural killer gene complex. Front Immunol. 2013;4:362.
Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol. 2011;11(10):645–57.
Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113(22):5488–96.
Fu B, Tian Z, Wei H. Subsets of human natural killer cells and their regulatory effects. Immunology. 2014;141(4):483–9.
Vossen MT, Matmati M, Hertoghs KM, Baars PA, Gent MR, Leclercq G, et al. CD27 defines phenotypically and functionally different human NK cell subsets. J Immunol. 2008;180(6):3739–45.
Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16(24):6019–28.
Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102(27):9571–6.
Assarsson E, Kambayashi T, Sandberg JK, Hong S, Taniguchi M, Van Kaer L, et al. CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J Immunol. 2000;165(7):3673–9.
Ohta N, Hiroi T, Kweon MN, Kinoshita N, Jang MH, Mashimo T, et al. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 alpha beta + NK1.1+ T cells for the development of small intestinal inflammation. J Immunol. 2002;169(1):460–8.
Correia MP, Costa AV, Uhrberg M, Cardoso EM, Arosa FA. IL-15 induces CD8+ T cells to acquire functional NK receptors capable of modulating cytotoxicity and cytokine secretion. Immunobiology. 2010;216(5):604–12.
Ohkawa T, Seki S, Dobashi H, Koike Y, Habu Y, Ami K, et al. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology. 2001;103(3):281–90.
Maglione JE, McGoldrick ET, Young LJ, Namba R, Gregg JP, Liu L, et al. Polyomavirus middle T-induced mammary intraepithelial neoplasia outgrowths: single origin, divergent evolution, and multiple outcomes. Mol Cancer Ther. 2004;3(8):941–53.
Ramanathan S, Gagnon J, Ilangumaran S. Antigen-nonspecific activation of CD8+ T lymphocytes by cytokines: relevance to immunity, autoimmunity, and cancer. Arch Immunol Ther Exp (Warsz). 2008;56(5):311–23.
Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114(12):2417–26.
Mueller YM, Petrovas C, Bojczuk PM, Dimitriou ID, Beer B, Silvera P, et al. Interleukin-15 increases effector memory CD8+ t cells and NK Cells in simian immunodeficiency virus-infected macaques. J Virol. 2005;79(8):4877–85.
Kajitani K, Tanaka Y, Arihiro K, Kataoka T, Ohdan H. Mechanistic analysis of the antitumor efficacy of human natural killer cells against breast cancer cells. Breast Cancer Res Treat. 2012;134(1):139–55.
de Kruijf EM, Sajet A, van Nes JG, Putter H, Smit VT, Eagle RA, et al. NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: an observational study. BMC Cancer. 2012;12:24.
Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106(6):1915–9.
Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209(13):2351–65.
Acknowledgements
We would like to acknowledge Dr. Gendler for the MT mice, Dr. Caliguiri for the IL-15 TG mice, Dr. Mossman for the MDA-231 cells and the Mayo Clinic for the MT cell line. The project was supported by the Canadian Breast Cancer Foundation (CBCF). Amy Gillgrass was supported by CIHR (2009-2011), followed by CBCF (2011-2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AG participated in the design of the study, carried out all experiments, analyzed and generated the figures and drafted the manuscript. MC and TK participated in carrying out the animal experiments and editing of the manuscript. AA conceived of the study and participated in its design. All authors read and approved the final manuscript.
Additional file
Additional file 1: Figure S1.
IFNγ production by CD8 T cells in the tumor and the spleen of IL-15 TG/MT and MT mice. Figure S2. IL-15 TG/MT tumors possess high levels of CD8+CD3+NK1.1+ T cells. Figure S3. Depletion efficiency in the spleen and tumor of IL-15 TG/MT mice when depleted with anti-NK1.1 (A) or anti-CD8α (B/C) antibody long term starting at 4 weeks of age (representative flow plots from n = 3 in each). Figure S4. Adoptive transfer of CD8 T cells from IL-15 TG/MT, MT or IL-15 TG mice does not protect from tumor formation.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Gillgrass, A.E., Chew, M.V., Krneta, T. et al. Overexpression of IL-15 promotes tumor destruction via NK1.1+ cells in a spontaneous breast cancer model. BMC Cancer 15, 293 (2015). https://doi.org/10.1186/s12885-015-1264-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1264-3