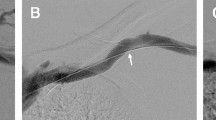
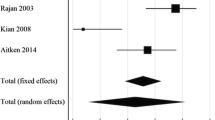
Abstract
Background
We evaluated restenosis rates at the cephalic arch after percutaneous angioplasty and stenting procedures in patients with brachial artery to cephalic vein arteriovenous fistula (BCAVF) hemodialysis access.
Methods
We used data from adult hemodialysis patients treated at a national network of 44 outpatient interventional facilities during Oct 2011–2015. We included data from patients with BCAVF who received an exclusive angioplasty, or stent with angioplasty, for treatment of cephalic arch stenosis and had ≥1 subsequent evaluation of the cephalic arch. Median percent restenosis per month at cephalic arch and days between encounters was calculated from the 1st index to 2nd procedure, and for up to 4 subsequent encounters. Analyses were stratified by intervention and device types.
Results
We identified a cohort of 3301 patients (mean age 62.2 ± 13.9 years, 58.5% male, 33.2% white race) with a BCAVF who had an angioplasty, or stent, at the cephalic arch for an index and ≥ 1 follow-up procedure. Between the 1st index to 2nd procedure, patients who received an angioplasty (n = 2663) or stent (n = 933) showed a median decrease of 18.9 and 16.5% in luminal diameter per month and a median time of 93 and 91 days between encounters, respectively. Restenosis and day rates were similar for standard versus high-pressure angioplasties. Bare metal stents showed 10.1 percentage point higher restenosis rate compared to stent grafts. Restenosis rates and time to restenosis were relatively consistent across subsequent encounters.
Conclusions
Findings suggest hemodialysis patients with a BCAVF who require an angioplasty or stent to treat a stenosis at the cephalic arch will have stenosis reformed at a rate of 18.9 and 16.5% per month after the first intervention, respectively. Findings suggest patients are at risk of having significant lesions at the cephalic arch within 3 months after the previous intervention.
Similar content being viewed by others
Background
In the United States, more than 785,000 people are diagnosed with kidney failure and require kidney replacement therapy (KRT) to sustain life [1]. About 62% of patients are treated by the KRT of hemodialysis, whereby blood is filtered three or more times per week using a vascular access. Dialysis access types include arteriovenous fistulae (AVFs), arteriovenous grafts (AVGs), or catheters. Of these options, AVFs are considered the preferred access given better function and lower infection and mortality rates compared to catheters and AVGs [2]. Also, studies have shown that AVFs demonstrate lower thrombosis and access failure rates than AVGs [3]. Among prevalent hemodialysis patients, about 66% use an AVF [1].
The three most common AVFs are the radio-cephalic fistula, brachial artery to cephalic vein fistula, and brachial artery to basilic vein transposed fistula. Although the first choice is the radio-cephalic forearm fistula, the brachial artery to cephalic vein fistula, or brachiocephalic fistula (BCAVF), is one of the most popular types for many reasons. Among several advantages is ease of creation, high maturation rates, and high flow rates. Disadvantages include higher rates of steal syndrome and symptomatic central venous stenosis [4].
With an increasing prevalence of BCAVF comes unique complications requiring treatment to avoid failure. For BCAVF, the typical site of stenosis is the cephalic arch [4,5,6,7]. Potential etiologies for stenosis and subsequent restenosis at the cephalic arch include increased flow in an outflow vein, external compression by fascia and pectoralis major, angulation, numerous valves in the outflow vein, and biochemical changes associated with kidney failure. The cephalic arch is typically a single channel joining with the axillary vein, yet variant anatomy such as a bifid arch, or abnormal termination point (internal or external jugular veins), can cause venous outflow obstruction and access malfunction [8]. It is likely a combination of multiple factors contribute to occurrence and recurrence of stenosis at this site.
To date, there are no large multicenter studies describing the treatment and effectiveness of percutaneous interventions at the cephalic arch in BCAVFs. Our study aimed to evaluate cephalic arch restenosis rates after percutaneous intervention, including transluminal balloon angioplasty and stenting.
Methods
Setting
We used data from a network of 44 outpatient vascular care and ambulatory surgery centers (Azura Vascular Care, Malvern, PA) across the United States between October-2011 through October-2015. This analysis was conducted under a protocol approved by New England Independent Review Board (IRB). The IRB determined this analysis of deidentified patient data was exempt and did not require informed consent per the United States Code of Federal Regulations 45CFR46 (Needham Heights, MA; NEIRB#WO-1-574-1). This study adhered with the Declaration of Helsinki.
Patient population
We included data from adult patients (age ≥ 18 years) with a brachiocephalic AVF (BCAVF) who: 1) were referred for evaluation of an access malfunction, 2) received an angioplasty or stent for treatment of stenosis at the cephalic arch, and 3) had ≥1 subsequent evaluation of the cephalic arch. After the initial referral, subsequent encounters were either a clinically timed evaluation scheduled by the interventionalist, or another referral for an access malfunction from the dialysis center (e.g. prolonged bleeding, high venous pressures, low access flows). We excluded data from patients who received a coinciding thrombectomy, pharmacomechanical thrombolysis, or embolization at the same encounter as an angioplasty/stent. The dataset also did not contain patients who had an access ligation or excision after initial referral visit.
Standard of care practices
Patient care was performed under the provider’s standard operating procedures by interventional radiologists, interventional nephrologists, and vascular surgeons who specialize in dialysis access care. A universal electronic medical record system was used to record encounters/procedures. Patients referred for evaluation of a malfunctioning AVF had historical and physical examination performed prior to intervention. During patient visits, the need for an angiogram was determined in a manner consistent with national guidelines, [9] and based on abnormalities identified during the physical examination, as well as complaints/subjective reports from the patient/dialysis center (e.g. increased pulsatility during physical exam combined with prolonged bleeding after hemodialysis). When angiogram was appropriate, intravenous fentanyl and midazolam were used for moderate sedation under nurse/anesthesiologist monitoring; propofol was also administered in rare instances. A combination of fluoroscopy with contrast and ultrasound was used for evaluation of fistulae. Percutaneous intervention(s) were performed on clinically significant lesions found to have stenosis with > 50% narrowing in luminal diameter as compared by the diameter of the nearest normal appearing vein found during two dimensional angiogram; these practices were consistent with national guidelines (i.e. Kidney Disease Outcomes Quality Initiative Clinical Practice Guideline for Vascular Access) [9]. The provider’s clinics did not use three-dimensional computed tomography angiography in standard practices, and therefore did not measure luminal area. The need for and timing of clinically timed evaluations after any given angioplasty or stent procedure was based on the medical judgment of the interventionalist.
Standard practice was typically to treat detected lesions with standard angioplasty (< 24 atm (ATM) rated burst pressure balloon) until stenosis was eliminated using inflation times based on operator discretion. When necessary, high-pressure angioplasty (> 24 ATM rated burst pressure balloon) were recommended to eliminate a lesion if resistant to standard angioplasty, or a known history of a lesion at the site requiring high-pressure angioplasty. Balloon size ranged from 4-to-14 mm in diameter. If a residual stenosis was identified, or if a stenosis was unresponsive to angioplasty, a stent or stent graft was placed. Stent size was recommended to be based upon comparison of the normal appearing vein with oversizing by 20–40% to accommodate for vein elasticity and position the stent. A post-stent deployment angioplasty could be performed to further expand the stent as clinically indicated.
Cephalic arch stenosis
Cephalic arch was defined as the portion of the cephalic vein within 5 cm of the confluence with the axillary vein, or larger outflow vein in instances of aberrant anatomy. Stenoses were assessed comparing the narrowest luminal diameter at the cephalic arch, as a percentage of the nearest normal appearing vein. Percent stenosis was documented before and after each intervention.
Analysis design
Analysis of restenosis rates by intervention type
We assessed data from patients who received either exclusively an angioplasty, or a stent with an angioplasty, for treatment of stenosis at the cephalic arch at each index procedure. The first encounter defined the 1st index procedure for patients who had an angioplasty without any stent placement. The first stent placement defined the 1st index procedure for patients who received a stent with an angioplasty.
For each intervention type (angioplasty or stent group), we calculated the per patient difference in the percent stenosis after the 1st index procedure (post-intervention) to the percent stenosis before the 2nd procedure (pre-intervention). We computed restenosis rates via the change in the percent stenosis per month from the 1st index to 2nd procedure. The equation below shows a mathematical description of the restenosis rate described above.
Recurrent stenosis and repeated interventions are common at the cephalic arch in BCAVFs, and alternative treatments may be worthwhile to be considered in appropriate cases where angioplasty has been ineffective. These include stents, with stent grafts appearing to have the most favorable outcomes in our analysis. Stent grafts have also been previously shown to be more effective in the treatment of lesions in AVFs and AVGs, as compared to bare metal stents and angioplasty alone [12,13,14,15, 21,22,23]. Stent grafts can be constructed from various materials (e.g. metals, plastics, woven polyester), but the differences between restenosis rates with stent graft types is speculative. Other alternative treatments include percutaneous drug eluting balloons, as well as other surgical procedures including cephalic arch turn-down and cut-down and patch angioplasty. These procedures were not evaluated in this study due to minimal-to-no use in the outpatient setting. With respect to drug eluting balloons, their use at the time of the study was not widespread secondary to cost of equipment and relatively little data on their outcomes during the time of the study.
Our study has several strengths, the most obvious of which includes the focus on exclusive interventions that afforded the ability to reasonably assess restenosis rates at a specific lesion site. In addition, the analysis included a large number of patients treated by various practitioner types in a broad geography. All these factors represent clinically relevant interventions and consequently, this data likely represents reproducible, real-world outcomes. However, this study cohort may not be fully generalizable and is not representative of the groups of patients who had unremarkable complications upon evaluation, or a mixture of interventions (e.g. thrombectomy and high-pressure angioplasty). We cannot rule out a potential bias by indication for angiogram. We did not have information on patients who did not require a subsequent intervention after their first procedure, which included more than 1200 patients who received only one measurement at the cephalic arch during a mixture of evaluations and/or intervention types. Furthermore, the provider’s centers did not routinely perform ultrasounds before inventions to provide further details. Nonetheless, the cohort included a reasonable number of patients with minimal to no restenosis after an index angioplasty or stent likely making the results generalizable to what occurs in treatment of access stenoses in BCAVFs. These results are not generalizable to all access failures, given the high potential for multifactorial causes including formation of a thrombus and blood vessels diverging into abnormal vascular channels. Further independent studies are needed to define the rates and risk factors of recurrent complications for those events linked to thrombosis of the access.
The retrospective nature of this study is an inherent weakness. We did not have access to data from the dialysis centers and several factors such as the time the BCAVF was in use may have the potential influence the results. Also, stenosis measurements may have varied by practitioner, although a small random sampling of cases with image review showed good agreement with reported degrees of stenosis. Given the large number of centers, inter-operator variability also poses a problem when evaluating the need for stenting, stent type, and general personal practice regarding stenting versus angioplasty alone. Also, the precise morphology of the stenosis, focal versus long segment versus multifocal, was not clearly specified. While morphology may play a role in the long-term success of endoluminal interventions, it is not clear stratifying the results based on morphology would have any impact. Furthermore, these models of recurrence are based on a linear progression of stenosis development warranting treatment. It is feasible that after numerous interventions, the rate of progression may change, either for better or worse.
Conclusions
In conclusion, the study demonstrated restenosis rates at the cephalic arch in hemodialysis patients with a brachiocephalic fistula requiring an angioplasty or stent were 18.9 and 16.5% per month after the first intervention, respectively. Restenosis rates appeared to be relatively consistent across subsequent procedures. Findings suggest patients are at risk of having significant lesions (> 50% stenosis) within 3 months after the previous intervention at the cephalic arch. When clinically indicated, use of stent grafts seemed to lessen recurrent stenosis, especially if used as the first stent. It appears prudent to have patients who develop a cephalic arch lesion return for an evaluation after approximately 3 months. At that time, a careful history and physical exam should be performed to determine if recurrence is present. If the assessment indicates such, imaging with possible intervention is recommended to maximize patency and functionality of a patient’s hemodialysis access, thereby prolonging life.
Availability of data and materials
The dataset used for this analysis is not publicly available. The data utilized was obtained from a private electronic medical record system, which is restricted to use by only authorized employees. Reasonable requests to access the study dataset might be considered under executed contractual agreements between Fresenius Medical Care and an external individual’s institution. Requests to access the dataset can be sent to the author J.W.L.
Abbreviations
- ATM:
-
Atmospheres
- AVF:
-
Arteriovenous fistula
- AVG:
-
Arteriovenous graft
- BCAVF:
-
Brachial artery to cephalic vein arteriovenous fistula
- IRB:
-
Independent Review Board
- KRT:
-
Kidney replacement therapy
References
United States Renal Data System. USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020.
Lok CE. Fistula first initiative: advantages and pitfalls. Clin J Am Soc Nephrol. 2007;2(5):1043–53.
Quencer KB, Oklu R. Hemodialysis access thrombosis. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S299–308.
Quencer KB, Arici M. Arteriovenous fistulas and their characteristic sites of stenosis. Am J Roentgenol. 2015;205(4):726–34.
Rajan DK, Bunston S, Misra S, Pinto R, Lok CE. Dysfunctional autogenous hemodialysis fistulas: outcomes after angioplasty--are there clinical predictors of patency? Radiology. 2004;232(2):508–15.
Turmel-Rodrigues L, Pengloan J, Baudin S, Testou D, Abaza M, Dahdah G, et al. Treatment of stenosis and thrombosis in haemodialysis fistulas and grafts by interventional radiology. Nephrol Dial Transplant. 2000;15(12):2029–36.
Rajan DK, Clark TW, Patel NK, Stavropoulos SW, Simons ME. Prevalence and treatment of cephalic arch stenosis in dysfunctional autogenous hemodialysis fistulas. J Vasc Interv Radiol. 2003;14(5):567–73.
Kian K, Asif A. Cephalic arch stenosis. Semin Dial. 2008;21(1):78–82.
Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75(4 Suppl 2):S1–S164.
Campos RP, Do Nascimento MM, Chula DC, Do Nascimento DE, Riella MC. Stenosis in hemodialysis arteriovenous fistula: evaluation and treatment. Hemodial Int. 2006;10(2):152–61.
Hammes M, Funaki B, Coe FL. Cephalic arch stenosis in patients with fistula access for hemodialysis: relationship to diabetes and thrombosis. Hemodial Int. 2008;12(1):85–9.
Shemesh D, Goldin I, Zaghal I, Berlowitz D, Raveh D, Olsha O. Angioplasty with stent graft versus bare stent for recurrent cephalic arch stenosis in autogenous arteriovenous access for hemodialysis: a prospective randomized clinical trial. J Vasc Surg. 2008;48(6):1524–31 1531 e1521–1522.
Rajan DK, Falk A. A randomized prospective study comparing outcomes of angioplasty versus VIABAHN stent-graft placement for cephalic arch stenosis in dysfunctional hemodialysis accesses. J Vasc Interv Radiol. 2015;26(9):1355–61.
D'Cruz RT, Leong SW, Syn N, Tiwari A, Sannasi VV, Singh Sidhu HR, et al. Endovascular treatment of cephalic arch stenosis in brachiocephalic arteriovenous fistulas: a systematic review and meta-analysis. J Vasc Access. 2019;20(4):345–55.
Miller GA, Preddie DC, Savransky Y, Spergel LM. Use of the Viabahn stent graft for the treatment of recurrent cephalic arch stenosis in hemodialysis accesses. J Vasc Surg. 2018;67(2):522–8.
Faxon DP, Weber VJ, Haudenschild C, Gottsman SB, McGovern WA, Ryan TJ. Acute effects of transluminal angioplasty in three experimental models of atherosclerosis. Arteriosclerosis. 1982;2(2):125–33.
Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, et al. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50(5):782–90.
Sivananthan G, Menashe L, Halin NJ. Cephalic arch stenosis in dialysis patients: review of clinical relevance, anatomy, current theories on etiology and management. J Vasc Access. 2014;15(3):157–62.
McLennan G. Stent and stent-graft use in Arteriovenous dialysis access. Semin Interv Radiol. 2016;33(1):10–4.
Hu H, Wu Z, Zhao J, Wang J, Huang B, Yang Y, et al. Stent graft placement versus angioplasty for hemodialysis access failure: a meta-analysis. J Surg Res. 2018;226:82–8.
Haskal ZJ, Trerotola S, Dolmatch B, Schuman E, Altman S, Mietling S, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med. 2010;362(6):494–503.
Kavan J, Kudlicka J, Malik J, Chytilova E, Lambert L, Slavikova M, et al. Treatment of failing arterio-venous dialysis graft by angioplasty, stent, and stent graft: two-years analysis of patency rates and cost-effectiveness. Exp Ther Med. 2019;18(5):4144–50.
Ng B, Fugger M, Onakpoya IJ, Macdonald A, Heneghan C. Covered stents versus balloon angioplasty for failure of arteriovenous access: a systematic review and meta-analysis. BMJ Open. 2021;11(6):e044356.
Acknowledgements
This article is in honor of the contributing author Melvin “Mel” Rosenblatt who passed away during the finalization of the analysis and manuscript composition. Mel was an impetus to this study and majorly contributed to the initial draft of this manuscript. Also, we would like to thank the interventionalists and care teams at Azura Vascular Care who captured the data used in this analysis during the provision of standard medical care.
Funding
The analysis and manuscript composition were internally supported by Fresenius Medical Care.
Author information
Authors and Affiliations
Contributions
Research idea and study design: R.N.R., M.R., Y.J., L.A.U., and J.W.L.; data acquisition: R.R., M.R., and N.M.; data analysis: Y.J. and L.A.U.; interpretation: R.N.R., M.R., Y.J., L.A.U., M.S., and J.W.L.; supervision or mentorship: R.N.R., L.A.U., M.S., and J.W.L.; All authors contributed to the manuscript drafting and/or revision. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This analysis was conducted under a protocol that was reviewed and approved by New England Independent Review Board (IRB) (Needham Heights, MA, United States; NEIRB#WO-1-574-1). The IRB approved the protocol under an Exempt Category since the analysis used deidentified patient data that did not include Protected Health Information and written/verbal informed consent was not required nor applicable per the Code of Federal Regulations (45CFR46) in the United States. This study adhered with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
All authors are employees of Fresenius Medical Care, or its subsidiary company Azura Vascular Care. L.A.U. has share options/ownership in Fresenius Medical Care. L.A.U., J.W.L. are an inventor on patent(s) in the field of dialysis. J.W.L. is a guest editor on the Editorial Board of Frontiers in Physiology.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with angioplasty
Additional file 2.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 3rd index to 4th visits among patients treated with angioplasty
Additional file 3.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with standard angioplasty
Additional file 4.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 3rd index to 4th visits among patients treated with standard angioplasty
Additional file 5.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with high-pressure angioplasty
Additional file 6.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 3rd index to 4th visits among patients treated with high-pressure angioplasty
Additional file 7.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with stent
Additional file 8.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 3rd index to 4th visits among patients treated with stent
Additional file 9.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with bare metal stent
Additional file 10.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 3rd index to 4th visits among patients treated with bare metal stent
Additional file 11.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with stent graft
Additional file 12.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with stent at the 1st index visit and angioplasty at the 2nd index visit
Additional file 13.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 3rd index to 4th visits among patients treated with stent at the 1st index visit and angioplasty at the 3rd index visit
Additional file 14.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with stent at the 1st index visit and standard angioplasty at the 2nd index visit
Additional file 15.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 3rd index to 4th visits among patients treated with stent at the 1st index visit and standard angioplasty at the 3rd index visit
Additional file 16.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with stent at the 1st index visit and high-pressure angioplasty at the 2nd index visit
Additional file 17.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 3rd index to 4th visits among patients treated with stent at the 1st index visit and high-pressure angioplasty at the 3rd index visit
Additional file 18.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with bare metal stent at the 1st index visit and angioplasty at the 2nd index visit
Additional file 19.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 3rd index to 4th visits among patients treated with bare metal stent at the 1st index visit and angioplasty at the 3rd index visit
Additional file 20.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 2nd index to 3rd visits among patients treated with stent graft at the 1st index visit and angioplasty at the 2nd index visit
Additional file 21.
Supplemental figure on distribution of restenosis rates (% decrease in lumen diameter per month) at the cephalic arch after the 3rd index to 4th visits among patients treated with stent graft at the 1st index visit and angioplasty at the 3rd index visit
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Razdan, R.N., Rosenblatt, M., Jiao, Y. et al. Cephalic arch restenosis rates in hemodialysis patients with brachiocephalic fistulae: a retrospective multicenter analysis of 3301 patients. BMC Nephrol 23, 109 (2022). https://doi.org/10.1186/s12882-022-02728-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02728-4