Abstract
Background
Differentiating between solitary spinal metastasis (SSM) and solitary primary spinal tumor (SPST) is essential for treatment decisions and prognosis. The aim of this study was to develop and validate an MRI-based radiomics nomogram for discriminating SSM from SPST.
Methods
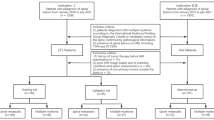
One hundred and thirty-five patients with solitary spinal tumors were retrospectively studied and the data set was divided into two groups: a training set (n = 98) and a validation set (n = 37). Demographics and MRI characteristic features were evaluated to build a clinical factors model. Radiomics features were extracted from sagittal T1-weighted and fat-saturated T2-weighted images, and a radiomics signature model was constructed. A radiomics nomogram was established by combining radiomics features and significant clinical factors. The diagnostic performance of the three models was evaluated using receiver operator characteristic (ROC) curves on the training and validation sets. The Hosmer–Lemeshow test was performed to assess the calibration capability of radiomics nomogram, and we used decision curve analysis (DCA) to estimate the clinical usefulness.
Results
The age, signal, and boundaries were used to construct the clinical factors model. Twenty-six features from MR images were used to build the radiomics signature. The radiomics nomogram achieved good performance for differentiating SSM from SPST with an area under the curve (AUC) of 0.980 in the training set and an AUC of 0.924 in the validation set. The Hosmer–Lemeshow test and decision curve analysis demonstrated the radiomics nomogram outperformed the clinical factors model.
Conclusions
A radiomics nomogram as a noninvasive diagnostic method, which combines radiomics features and clinical factors, is helpful in distinguishing between SSM and SPST.
Similar content being viewed by others
Introduction
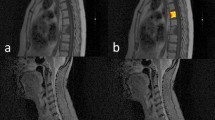
The spine is a common site for metastatic disease and primary tumors, with metastatic disease being much more frequent [1]. Nearly 70% of patients with cancers develop bone metastases, and approximately 40% of cancer patients occur spinal metastases during the course of their disease [2]. Typically, metastases appear as multifocal lesions in the axial skeleton, which can be suggestive for diagnosis. However, when metastasis presents as a solitary lesion, imaging features of solitary spinal metastasis (SSM) and solitary primary spinal tumor (SPST) frequently overlap, making it difficult to distinguish by traditional imaging practice [3, 4]. In addition, although tumor history is suggestive for the diagnosis of metastasis, its help is limited [5, 6]. The treatment decisions and prognosis are noticeably different depending on the diagnosis of spinal disease [7, 8]. Therefore, differentiating SSM from SPST is essential for the prognostic evaluation of the patients and the selection of appropriate treatment.
Several studies [9, 10] presented that positron emission tomography/computed tomography (PET/CT) could provide both functional and anatomic information, and is an excellent functional imaging method to identify spinal metastasis. However, this examination may not be widely available for clinical use due to its expensive price [11]. In addition, most investigators showed that percutaneous biopsy is a precise diagnostic method for spinal disease, however, it could result in complications such as hematoma, infection, and organ damage, so not all patients can undergo biopsy [5, 12]. Therefore, an efficient and non-invasive approach to discriminate SSM from SPST is urgent.
Recently, radiomics, as an emerging method of medical image analysis, can extract and analyze quantitative features from radiographic images [13, 14]. And radiomics has been shown to be helpful in tumor detection, diagnosis, prognostic assessment, prediction of treatment response, and monitoring of disease status [15]. Sun et al. [16] demonstrated that radiomics can differentiate between benign and malignant spinal tumors. In addition, previous studies have also assessed the ability of radiomics to differentiate between spinal metastases and other pathological spinal lesions [17,18,Full size image
Table 2 summarizes the performance of the three models on the training and validation sets. ROC curves of the three models for both the training and validation sets are shown in Fig. 6. The radiomics nomogram model markedly outperformed the clinical factors model in both the training set (AUC 0.980 vs. 0.807, p < 0.01) and validation set (AUC 0.924 vs. 0.679, p = 0.020). However, there was no statistically significant difference between the diagnostic performance of the radiomics nomogram and that of the radiomics signature model.
The decision curve analysis (Fig. 7) further indicated that the radiomics nomogram provided a higher net benefit and predictive ability in differentiating SSM from SPST than the clinical factors model.
Decision curve analysis for the prediction models in the validation cohort. The y-axis measures the net benefits, while the x-axis represents the threshold probability. The red line, blue line, and green line represent the net benefit of the combined model, the clinical factor model, and the radiomics signature, respectively. "All" and "none" are two reference lines. The gray line indicates the hypothesis that all tumors are SSM. The horizontal black line indicates the hypothesis that no tumors are SSM. The DCA analysis showed that the radiomics nomogram was more beneficial than the clinical factors model