Abstract
Background
Leadless pacemaker was a promising innovation than traditional transvenous pacemaker, the procedural complications were prone to be bleeding-related. However, very few reports also concerned about the thrombus formation during the procedure.
Case presentation
A hemodialysis patient with diabetic gangrene of right foot suffered from catheter-related thrombosis during leadless pacing, resulting in failure of recapture the pacemaker. A low activated clotting time (ACT) level of 104 s confirmed the insufficiency of anticoagulation. Finally, the whole delivery catheter had to be removed from the delivery sheath, another new pacemaker system was applied and successfully implanted after adjusting the ACT level to 248 s.
Conclusion
Catheter-related thrombosis could be a large obstacle for leadless pacemaker implantation. In addition to routine anticoagulation, ACT monitoring might be necessary during the procedure.
Similar content being viewed by others
Background
Leadless pacemaker was a promising innovation than traditional transvenous pacemaker with its miniaturized profile and superior safety. The procedural complications such as venous site hemorrhage, arteriovenous fistulas and pericardial effusion had been studied [1], which were prone to be bleeding-related. However, very few reports also concerned about the thrombus formation during the procedure. We presented a case for the first time that a hemodialysis patient with diabetic gangrene of right foot suffered from catheter-related thrombosis, resulted in difficulty of recapture and withdrawal the pacemaker. A low activated clotting time (ACT) level of 104 s confirmed the insufficiency of anticoagulation.
Case presentation
A 68-year-old man, who was 175 cm height and 75 kg weight with a body mass index (BMI) of 24.5, suffered from syncope for several times, the ECG showed paroxysmal atrial fibrillation with long RR intervals for up to 9.3 s (Fig. 1). The echocardiography showed mild-to-moderate tricuspid regurgitation, normal ventricular wall motion and normal left ventricular ejection fraction. The medical history included end-stage renal disease (ESRD) requiring hemodialysis for 6 years, diabetic gangrene of right foot for 5 months. Indication for pacemaker implantation was established according to the ECG results, in order to prevent transvenous pocket inflammation or systemic inflammation from the gangrenous foot, the Micra leadless pacemaker (Medtronic, Inc. Minneapolis, USA) was recommended and the informed consent was approved by the patient.
The right femoral vein was successfully punctured and inserted with a 6 French sheath, and a bolus of 3000 IU unfractionated heparin (UFH) was intravenous administrated at the beginning of the procedure. The access site was gradually dilated by 8- and 12-French dilators, and a 23-French delivery sheath was introduced into the inferior vena cava, then the pacemaker delivery catheter was connected to the sheath. All delivery devices were flushed and connected with heparinized saline properly. The Micra pacemaker was advanced and conducted into the mid-septum of right ventricle and the fluoroscopic location was acceptable. However, electrical measurement showed a low sensing amplitude of only 2.8 mV, suggesting that the pacemaker needed to be repositioned again.
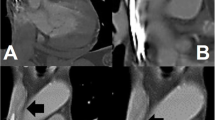
For recapture, the tether connected to the handle was pulled with constant tension and the pacemaker was closed to the recapture cone, different fluoroscopic angles were viewed to confirm the devices were coaxial. Since then the delivery cup could not be advanced due to great resistance, the pacemaker was not able to be withdrew into the catheter (Fig. 2, Additional file 1). Finally, the whole delivery catheter had to be removed from the delivery sheath (Fig. 2, Additional file 2).
a Fluoroscopic RAO angle showed the pacemaker was coaxial with the delivery cup but could not be pulled back. b Fluoroscopic LAO angle showed the recapture of pacemaker failed again. c The whole delivery catheter was withdrawing back into the delivery sheath. d The pacemaker was recaptured with great resistance
Luckily no valvular or myocardial tissues were found at the tip of the catheter, instead the lumen was fully filled with heavy thrombi (Fig. 3). An instant ACT level of 104 s revealed that procedural anticoagulant therapy was insufficient, thus, additional 4000 IU (a total amount of 7000 IU) UFH was administrated immediately and the ACT level turned to 248 s 10 min later. After being removed all the thrombi the structure and mobility of the delivery catheter recovered (Fig. 4), which in turn proved that the failure of recapture was caused by the thrombi within the lumen. For preventing thromboembolism, a new pacemaker system was applied and successfully implanted. The final electrical measurement was satisfied: pacing threshold was 0.5 V at 0.24 ms, sensing amplitude was 7.9 mV, and pacing impedance was 560 Ω. The final ACT level was 204 s.
Discussion
An increasing number of patients will no doubt benefit from the new pacing technology. The procedure seems to be easier and safer than traditional pacing with over 95% successful rate. The key point of the implantation is to settle the pacemaker with suitable electrical parameters, which sometimes requires retrieving and redeploying the device. In most of the time the retrieval is without difficulty unless the nearby myocardial tissues are hooked by the pacemaker. In this case, we proved that thrombosis within the catheter could also hinder the movement of delivery cup or somehow affect the coaxality and led to the failure of recapture.
According to the guideline on percutaneous coronary intervention UFH is the preferred anticoagulant [2], the recommended dose is 70–100 U/kg intravenous and need not to be adjusted in CKD patients. But it is not clear whether routine anticoagulation is mandatory during leadless pacemaker implantation. Thrombosis s very rare [3] as compared with the bleeding complications, some experienced centers recommended a dose of 3000–5000 IU UFH intravenous without monitoring the ACT [4] and continuously flushing the delivery devices with heparinized saline. But in hemodialysis patients the data was lacking.
In our case the patient was at both high thromboembolic and bleeding risks with 4 points of CHA2DS2VASc score and 3 points of HASBLED score, which made the strategy for anticoagulation challenging. Considering the hemodialysis background UFH seemed to be the optimal anticoagulant for the patient. But apparently 3000 IU UFH was insufficient according to the first ACT level, additional 4000 IU UFH turned the level into 248s which almost reached the ideal ACT level during coronary intervention (between 250s and 300s) and successfully prevented another thrombosis complication. This could be an important finding: in complex situations, ACT levels should be measured in order to evaluate the anticoagulant effects in time. But the proper ACT ranges for leadless pacemaker implantation were still unknown and needed to be discussed further.
The safety during periprocedural period and the 1-year follow-up was promising for hemodialysis patient [5]. The patient also recovered well and was discharged 3 days later. Post-procedural echocardiography showed no evidence of pericardial effusion.
In conclusion, Catheter-related thrombosis could be a large obstacle for leadless pacemaker implantation. In addition to routine anticoagulation, ACT monitoring might be necessary during the procedure, and the appropriate ACT levels deserve further study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its Additional files 1, 2].
Abbreviations
- ACT:
-
Activated clotting time
- BMI:
-
Body mass index
- ESRD:
-
End-stage renal disease
- UFH:
-
Unfractionated heparin
References
Lee JZ, Mulpuru SK, Shen WK. Leadless pacemaker: Performance and complications. Trends Cardiovasc Med. 2018;28(2):130–41.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
Cipolletta L, Volpato G, Biffi M, Capucci A. An indissoluble knot: an unexpected troubleshooting during Micra implantation. Pacing Clin Electrophysiol PACE. 2019;42(6):747–8.
Martínez-Sande JL, García-Seara J, Rodríguez-Mañero M, Fernández-López XA, González-Melchor L, Redondo-Diéguez A, et al. The micra leadless transcatheter pacemaker. Implantation and mid-term follow-up results in a single center. Revista espanola de cardiologia (English ed). 2017;70(4):275–81.
El-Chami MF, Clementy N, Garweg C, Omar R, Duray GZ, Gornick CC, et al. Leadless pacemaker implantation in hemodialysis patients: experience with the micra transcatheter pacemaker. JACC Clin Electrophysiol. 2019;5(2):162–70.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
QC analyzed and interpreted the data. YM and CZ performed the operation. LZ was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of the sixth medical center of Chinese PLA general hospital.
Consent for publication
The written informed consent to publish this information was obtained from the individual.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Failure of withdrawing the pacemaker into the catheter, the delivery cup could not be advanced due to great resistance.
Additional file 2. The whole delivery catheter was removed from the delivery sheath.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Q., Ma, Y.J., Zhang, C.H. et al. Insufficient procedural anticoagulation during leadless pacing led to catheter-related thrombosis in a hemodialysis patient. BMC Cardiovasc Disord 21, 502 (2021). https://doi.org/10.1186/s12872-021-02318-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02318-6