Abstract
Background
Low-molecular-weight glutenin subunits (LMW-GS), encoded by Glu-3 complex loci in hexaploid wheat, play important roles in the processing quality of wheat flour. To date, the molecular characteristics and effects on dough quality of individual Glu-3 alleles and their encoding proteins have been poorly studied. We used a Glu-A3 deletion line of the Chinese Spring (CS-n) wheat variety to conduct the first comprehensive study on the molecular characteristics and functional properties of the LMW-GS allele Glu-A3a.
Results
The Glu-A3a allele at the Glu-A3 locus in CS and its deletion in CS-n were identified and characterized by proteome and molecular marker methods. The deletion of Glu-A3a had no significant influence on plant morphological and yield traits, but significantly reduced the dough strength and breadmaking quality compared to CS. The complete sequence of the Glu-A3a allele was cloned and characterized, which was found to encode a B-subunit with longer repetitive domains and an increased number of α-helices. The Glu-A3a-encoded B-subunit showed a higher expression level and accumulation rate during grain development. These characteristics of the Glu-A3a allele could contribute to achieving superior gluten quality and demonstrate its potential application to wheat quality improvement. Furthermore, an allele-specific polymerase chain reaction (AS-PCR) marker for the Glu-A3a allele was developed and validated using different bread wheat cultivars, including near-isogenic lines (NILs) and recombinant inbred lines (RILs), which could be used as an effective molecular marker for gluten quality improvement through marker-assisted selection.
Conclusions
This work demonstrated that the LMW-GS allele Glu-A3a encodes a specific LMW-i type B-subunit that significantly affects wheat dough strength and breadmaking quality. The Glu-A3a-encoded B-subunit has a long repetitive domain and more α-helix structures as well as a higher expression level and accumulation rate during grain development, which could facilitate the formation of wheat with a stronger dough structure and superior breadmaking quality.
Similar content being viewed by others
Background
Wheat (Triticum aestivum L., 2n = 6x = 42, AABBDD), as a complex allohexaploid species, is one of the most important crops widely cultivated across the world. Wheat grains contain about 10–15% proteins, and are one of the richest protein sources in the human diet. It is well known that wheat breadmaking quality is largely determined by the seed storage proteins present in the grain endosperm, which mainly consist of polymeric glutenins and monomeric gliadins [1],[2]. The polymeric glutenins are further subdivided into high-molecular weight glutenin subunits (HMW-GS) and low-molecular-weight glutenin subunits (LMW-GS) according to their mobilities on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, which determine their dough elasticity, viscosity, and strength [2],[3].
LMW-GS can be separated into three groups, the B, C, and D subunits, based on their electrophoretic mobilities on an SDS-PAGE gel. Genetic analysis showed that these subunits are encoded by the Glu-A3, Glu-B3, and Glu-D3 loci on the short arms of the chromosomes 1A, 1B, and 1D, respectively [4],[5]. Some components were also found to be encoded by genes on the short arms of the group 6 and 7D chromosomes [6]. Based on their N-terminal amino acid sequences, LMW-GS are classified into three subclasses, LMW-m, LMW-s, and LMW-i types, according to the first amino acid residue of the mature protein: methionine, serine, and isoleucine, respectively [6]. The LMW-s type subunit seems to be predominant [7],[8]. Typically, the N-terminal amino acid sequence is SHIPGL- in LMW-s type subunits, while LMW-m type subunits have various N-terminal sequences such as METSHIGPL-, METSRIPGL-, and METSCIPGL- [9]-[11]. The LMW-i type subunit, first reported by Pitts et al. [12], lacks the N-terminal domain and starts directly with the repetitive region of ISQQQQ- after the signal peptide. Although the typical N-terminal domain is absent, LMW-i type subunits can be expressed normally, similar to LMW-m and LMW-s, in the wheat endosperm [13],[14]. Most LMW-GSs possess eight cysteine residues, although their positions vary in the different types of subunits, which plays important roles in the formation of intra- and inter-molecular disulfide bonds in the gluten macropolymer [14].
Compared to the Glu-1 loci encoding HMW-GS, Glu-3 loci exhibit more extensive allelic variations that are closely related to gluten quality. Early work by Gupta and Shepherd [15] identified and named six alleles at Glu-A3, nine alleles at Glu-B3, and five alleles at Glu-D3 loci in common wheat. Recently, 14 unique LMW-GS genes in the wheat cultivar **aoyan 54 were identified, four of which were located at Glu-A3, three at Glu-B3, and seven at Glu-D3, based on bacterial artificial chromosome (BAC) library screening and proteomics analysis [16]. The results from a set of Aroona LMW-GS near isogenic lines (NILs) showed that the Glu-A3 locus has two m-type and 2–4 i-type genes [17]. Analysis of the micro-core collections (MCC) of Chinese wheat germplasm identified more than 15 LMW-GS genes from individual MCC accessions, 4–6 of which were located at the Glu-A3 locus [18].
Since extensive allelic variations are present at Glu-3 loci, it is generally difficult to accurately determine the functional properties of individual alleles in different genotypes. To date, the main method used to investigate the effects of different Glu-3 alleles on dough quality has involved determination of their effects and ranks in NILs. Earlier research on the durum wheat NILs Lira 42 and Lira 45 showed that the LMW-2 type subunit in Lira 45 had significantly greater beneficial effects on gluten strength and breadmaking quality than the LMW-1 subunit in Lira 42 [19]. In bread wheat, Glu-A3d possesses three active LMW-GS genes and produces the highest Zeleny sedimentation value (ZSV) and Extensograph maximum resistance (Rmax) [17]. Other reports also showed that the Glu-A3d allele had a superior effect on dough strength [20]-[22]. Recent work on a set of Aroona NILs showed that Glu-A3b contributed to a longer midline peak time (MPT) and better raw white Chinese noodle (RWCN) color [23]. Despite the large number of studies performed on the functions of Glu-3 alleles, more comprehensive and in-depth analyses on the structures and functions of the individual alleles at Glu-3 loci are still lacking.
In the current work, we conducted the first comprehensive investigation on the molecular characteristics and functional properties of the LMW-GS allele Glu-A3a by using a Glu-A3 deletion line in the Chinese Spring (CS) wheat cultivar in combination with various proteomics and molecular biology approaches. Our results demonstrate that the deletion of Glu-A3a significantly reduces wheat dough strength and breadmaking quality. In addition, we demonstrated that Glu-A3a results in a longer repetitive domain and more α-helices in the encoded subunit, as well as a higher expression level and accumulation rate during grain development, which could help to improve the formation of a stronger dough structure and superior quality.
Results
Identification and characterization of seed proteins in CS and the Glu-A3deletion line CS-n
A Glu-3 deletion line of CS was screened and developed in our laboratory, and named CS-n. Compared to CS, the morphological characteristics of plants, spikes, and seeds, as well as the growth and development traits of CS-n showed no significant differences (Additional file 1: Figure S1, Additional file 2: Figure S2, and Additional file 3: Table S1). The grain protein compositions of CS and CS-n were identified by using various proteome approaches (Figure 1 and Additional file 4: Figure S3). The results indicated that CS-n showed the same albumin and globulin compositions as CS, while gliadins displayed minor differences between CS-n and CS; only one gliadin band obtained by acidic polyacrylamide gel electrophoresis (A-PAGE) was absent in CS-n (Additional file 4: Figure S3).
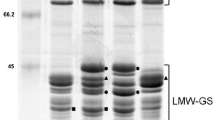
Identification of Glu-A3a in Chinese Spring (CS) and Glu-A3 deletion line CS-n. a. SDS-PAGE: the Glu-A3a encoded B-subunit as well as LMW-GS and HMW-GS were indicated. b. 2-DE: two differentially expressed protein spots between CS and CS-n encoded by Glu-A3a were marked by ① and ②. c. RP-UPLC: two protein peaks encoded by Glu-A3a in CS as well as LMW-GS and HMW-GS were indicated.
Glutenin subunits identified by SDS-PAGE indicated that HMW-GS in CS-n were the same as those in CS (N, 7 + 8, 2 + 12), and most LMW-GS bands were also identical, except that one clear B-type LMW-GS encoded by Glu-A3a was absent in CS-n (Figure 1a). Two-dimensional electrophoresis (2-DE) analysis revealed that Glu-A3a encodes two proteins (spots 1 and 2 in Figure 1b), which were further determined to be one LMW-i type subunit by liquid chromatography-tandem mass spectrometry (LC-MS/MS), as shown in Table 1. Reversed-phase ultra-performance liquid chromatography (RP-UPLC) analysis further confirmed that Glu-A3a encodes two protein components (peaks 1 and 2 in Figure 1c), which were eluted at 15.5 min and 16 min, respectively. Both peaks accounted for 22.58% of the total LMW-GS in CS.
To obtain the accurate molecular mass of the Glu-A3a-encoded B-subunit, the expected protein band on the SDS-PAGE gel indicated in Figure 1a was collected and then analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). As shown in Additional file 5: Figure S4, the Glu-A3a-encoded LMW-GS B-subunit was easily identified, and its molecular mass was determined to be 41,701.2 Da.
Confirmation of Glu-A3adeletion in CS-n with a sequence-tagged site polymerase chain reaction (STS-PCR) marker
To further confirm the deletion of the Glu-A3 locus in CS-n, a pair of STS primers developed from the single nucleotide polymorphisms (SNPs) in Glu-A3 allelic variants [24] were used to amplify the Glu-A3a gene. As shown in Figure 2, one specific PCR product of 529 bp was amplified in CS, the CS-1Sl/1B substitution line, the CS-1Sl addition line, and Aroona, which contain the Glu-A3a allele, whereas no such fragments were obtained in the other materials without Glu-A3a, such as CS-n. The specific amplified 529-bp fragment was sequenced, and the sequence was the same as those from the upstream 140–395 bp of the Glu-A3a-coding sequence shown in Additional file 6: Figure S5. Thus, these results confirmed that the Glu-A3 locus was deleted in CS-n.
Identification of Glu-A3a by STS-PCR markers. 1. CS ( Glu-A3a ), 2. CS-n; 3. CS-1Sl/1B; 4. CS 1Sl addition line; 5. Aroona-A3a (Glu-A3a); 6. Aroona-A3b (Glu-A3b); 7. Aroona (Glu-A3c); 8. Aroona-A3d (Glu-A3d); 9. Aroona-A3e (Glu-A3e); 10. Aroona-A3f (Glu-A3f); 11. Glenlea (Glu-A3g); 12. CB037A. M. molecular mass marker: 2000 bp, 1500 bp, 1000 bp and 500 bp. Glu-A3a fragment with 529 bp was arrowed.
Comparison of gluten quality properties between CS-n and CS
Dough strength and breadmaking quality testing showed that the main gluten quality parameters in CS-n were significantly reduced compared to those of CS (Tables 2 and 3). In general, flour yield, water absorption, final viscosity, and peak viscosity between CS-n and CS showed no apparent differences. However, deletion of Glu-A3a in CS-n increased the ash content by 15.39%. Ash content is an important indicator of flour quality, which has a moderately negative effect on noodle color [25]. In addition, the deletion of Glu-A3a in CS-n resulted in a significant decrease of the gluten index (4% reduction) and an increase in the flour falling number (5.05% increase), as shown in Table 1. The gluten index was shown to have a positive relationship with strong dough property [26].
Farinograph analysis indicated that development time, stability time, tolerance index, and farinograph quality number in CS-n were significantly lower than those in CS (Table 2). These properties led to a decrease in loaf volume of CS-n from 760 to 735 cm3 (Table 2 and Figure 3). Bread texture analysis showed that the hardness and resilience of bread in CS were superior to those in CS-n. Further cell size analysis of the bread demonstrated that the quality in CS-n was significantly reduced (Table 3). For example, wrapper length, slice brightness, and wall thickness of CS-n bread slices were much lower than those of CS. The cell diameter and elongation in CS-n were also reduced as a result of Glu-A3a deletion.
Molecular characteristics of the LMW-GS allele Glu-A3a
To further understand the molecular mechanisms underlying the significant effects of Glu-A3a on gluten and breadmaking quality, the complete coding sequence of Glu-A3a was amplified and sequenced by allelic-specific (AS) PCR. Based on the previously characterized Glu-A3 genes, a pair of specific primers (A3-F and A3-R) for the Glu-A3 locus was designed and used to amplify the Glu-A3a allele from CS. As shown in Additional file 7: Figure S6, a single band of approximately 1100 bp was obtained from CS, whereas no product was amplified from CS-n. Since most of the complete coding sequences of LMW-GS genes vary in length between 909 and 1167 bp [6],[27]-[29], the size of the amplified band corresponded well to the known LMW-GS gene sizes. After sequencing of the amplified product, a complete open reading frame of 1134 bp was obtained. Sequence alignment showed that the cloned gene had no internal stop codons and contained typical structural features of LMW-GS, and therefore was named as Glu-A3a (Additional file 7: Figure S6). After searching the GenBank database, we found that the cloned Glu-A3a gene had the same sequence as GluA3-11 from cultivar Aroona-A3a (GenBank accession number FJ549928). The deduced amino acid sequence of Glu-A3a showed the presence of an isoleucine as the first amino acid residue in the N-terminal of the mature protein, indicating that it belongs to the LMW-i type subunit [6].
The complete coding sequence of Glu-A3a was aligned with 15 other known LMW-i type genes to detect SNP and insertion/deletion (InDel) variations, and the results are listed in Table 4. These LMW-i genes originated from different Triticum species, including T. aestivum and T. dicoccoides. Six SNPs at different positions, resulting from G-A or C-T transitions and two deletions at nucleotides 81 and 854, were identified in Glu-A3a. Six SNPs could produce amino acid substitutions, and thus are considered nonsynonymous SNPs.
The deduced amino acid sequence of Glu-A3a had 376 amino acid residues with a predicted molecular mass of 41,346.1 Da, corresponding well to that determined by MALDI-TOF-MS (41,701.2 Da). Multiple alignment of the deduced amino acid sequences of Glu-A3a with the other 14 LMW-i type subunits (Figure 4) showed that all have conserved signal peptides and four domains in the mature protein sequences, including a repetitive domain, cysteine-rich region, glutamine-rich region, and C-terminal conservative region, as reported by Cassidy et al. [27]. Similar to other LMW-i type subunits, the Glu-A3a-encoded subunit contained eight cysteine residues at relatively conserved positions (Additional file 8: Table S2). It is speculated that the first and seventh cysteines of the LMW-GS form the inter-molecular disulfide bond, while the rest form three intra-molecular disulfide bonds [30],[31].
Multiple alignment of the deduced amino acid sequences of Glu-A3a and other 14 LMW-i glutenin genes. These genes including GenBank number AB062877 [14], AY542896 [13], DQ307386 [32], EU189087 [33], EU594335 and EU594336 [34], FJ549929, FJ549931, FJ549932 and FJ449933 [24], FJ876819 (Han, 2009), GQ870245, GQ870249 [35] and GU942731 [36]. Signal represents signal peptide (I), repetitive domain (II) and three sub-regions of C-terminal domain were indicated, respectively. The first amino acid residue of the mature proteins and cysteine residues were highlighted by black box and red shading, respectively. Deletions were indicated by dashes. Polyglutamine stretches were indicated by broken line frames.
The number of repeats present in the repetitive domain is mainly responsible for the length variation and the general hydrophilic character of LMW-GS [30]. The Glu-A3a-encoded subunit contained the typical repeat motif of LMW-GS: P1–2FP/SQ2–6. Our results showed that Glu-A3a has a rather large and regular repeated sequence domain that includes a high proportion of glutamine residues (about 46%) in the repeats (consensus sequence PPFSQQQQ), and two polyglutamine stretches with 11 and 12 continuous glutamine residues in the repetitive and C-terminal domains, respectively. Repeat motif numbers in LMW-i subunits are much higher than those in the LMW-m and LMW-s subunits, ranking them the longest protein subunits among all Glu-3 loci.
Secondary structure and function prediction of the Glu-A3a-encoded protein
The secondary structures of the Glu-A3a-encoded protein (FJ549928) and five other LMW-i type subunits from bread wheat (AY724436, AY724437, AY263369, AY831866, and AY542896) were predicted by the PSIPRED server, as shown in Table 5. The results showed that the α-helices and β-strands were dispersed in the normal configuration in C-terminal I and were highly conserved in C-terminal III. FJ549928 contained seven α-helices, mainly located at the C-terminal, and one β-strand dispersed in the conserved C-terminal region. Thus, the number of α-helices in FJ549928 was much higher than that of the other five subunits, which contain only 0–3 α-helices. For example, the LMW-i type glutenin subunit AY542896, assigned to the 1A chromosome, only has one α-helix, which was confirmed to co-migrate with the LMW-50 subunit that plays an important role in determining good quality characteristics of Glenlea [13] and the XYGluD3-LMWGS1 subunit (AY263369), with only 3 α-helices, is also considered to have a positive effect on dough quality [37].
Phylogenetic analysis of Glu-A3aand other LMW-GS genes
A homology tree was constructed to reveal the phylogenetic relationships among 25 LMW-GS genes at Glu-3 loci from different species and genomes through nucleotide sequence alignment of their coding regions using MEGA5 software (Figure 5). These sequences comprised 21 LMW-GS genes from different genomes of Triticum diploid, tetraploid, and hexaploid species. The phylogenetic tree displayed two clear branches, which corresponded well to distinguishing the LMW-i type from the LMW-m and LMW-s type subunits. This demonstrated that LMW-i type genes have undergone greater divergence during evolution compared to LMW-s and LMW-m genes, as previously reported [38],[39]. Sine LMW-m and LMW-s type subunit genes generally show higher consistency, they showed close phylogenetic evolutionary relationships. Glu-A3a showed a closer relationship with other LMW-i type genes from common wheat. All of the LMW-i type subunit genes from common wheat and related species shared higher sequence identity, indicating their high evolutionary conservation.
Homology tree constructed based on the coding regions of 21 LMW-GS genes. 21 LMW-GS genes named AB062876, AB062877 and AB062878 [14], AB262661 (Takeuchi T, 2006), AB119007 and AB164415 [40], AY453158 and AY453159 [41], AY585355 [42], DQ307389, DQ307387 and DQ345449 [39], DQ457416 [55]. For example, the over-expressed HMW-GS 1Bx7OE has positive effects on dough strength [53],[56]. In addition, the accumulation rates vary between different groups of proteins, suggesting differential regulation of protein biosynthesis and different quality performance. In particular, the wheat biotype with superior HMW-GS 5 + 10 subunits accumulated larger polymers more quickly than the biotype with poor allelic subunits 2 + 12 [54].
The B-subunits of LMW-GS are the most abundant and have the greatest impact on wheat processing qualities [6]. In this work, RP-UPLC analysis revealed a higher expression level and greater proportion of Glu-A3a-encoded B-subunits, accounting for more than 22% of the total LMW-GS in CS (Figure 1c), indicating its major contribution to LMW-GS synthesis and its important roles in determining dough quality. A recent study also found that higher numbers of active LMW-GS genes at the Glu-A3 and Glu-D3 loci in ** grains of hexaploid wheats. J Exp Bot. 1996, 47 (9): 1377-1385. 10.1093/jxb/47.9.1377." href="/article/10.1186/s12870-014-0367-3#ref-CR54" id="ref-link-section-d142980475e3962">54],[57]. LMW-GS, HMW-GS, and ω-gliadins can be detected by gel electrophoresis as early as 7 DPA [54], and 10–18 DPA represents the key stage of storage protein synthesis [58]. In the present study, Glu-A3a transcripts demonstrated an up-down expression pattern during grain development, and the highest expression level occurred at 14 DPA (Figure 7a), similar to a previous report [55]. Thus, the Glu-A3a-encoded B-subunit has a higher accumulation rate during grain development similar to HMW-GS 5 + 10 [54], which could improve the conformation of the regular gluten structure. Some important genes related to storage protein folding and synthesis, such as protein disulfide isomerase (PDI) and binding protein (BiP) genes, generally have higher expression levels at the early grain developmental stages. For instance, the PDI genes PDIL1-1 and PDIL2-1, which are involved in disulfide bond formation, displayed a peak expression level in the early stages (about 10–15 DPA) of grain development [58]. The higher accumulation rate of the Glu-A3a-encoded B-subunit was accompanied by higher expression levels of the genes involved in storage protein synthesis and assembly during early grain development, suggesting that this subunit could improve the conformation of gluten macropolymers (GMP) and result in superior dough quality.
Potential application of Glu-A3ain wheat quality improvement through molecular marker-assisted selection
Characterization of the allelic variations of LMW-GS is important for improvement of wheat-processing quality. Some allelic variations of LMW-GS have greater positive effects on dough properties than others [3],[45],[60]. Marker-assisted selection is an effective supplement to conventional breeding practices. For LMW-GS, because of the low resolution of traditional SDS-PAGE and the tedious operation procedures involved in 2-DE, development of different markers is important for the study and application of target subunits.
Recently, with increasing numbers of LMW-GS alleles being cloned and sequenced from common wheat, different molecular markers have been developed to rapidly screen and select desirable Glu-3 alleles. Zhang et al. [41] developed a set of markers that can be used to discriminate the alleles Glu-A3a, b, c, d, e, f, and g. Long et al. [61] classified 69 LMW-GS genes registered in GenBank into nine groups and established nine group-specific primer sets to identify each group. Ikeda et al. [62] developed 12 specific PCR markers to distinguish 12 groups of LMW-GS genes in the wheat cultivar Norin 61. Ten allele-specific STS markers for Glu-D3 were developed by Zhao et al. [10: Table S3.
Identification of seed proteins
Protein extraction, A-PAGE, SDS-PAGE and RP-UPLC
According to the solubility in a series of solvents, grain albumins, globulins, gliadins and glutenins were extracted according to the established methods [28],[34],[39],[76],[77].
Expression of the cloned LMW-GS gene in E. coli
The gene cloned was re-amplified to remove the signal peptides by designing a new pair of primers CS-F (5’-GGGCATATGATTTCACAGCAACAA-3’) and CS-R (5’-CTCGAGTCAGTAGACACCAACTCCGATG-3’), NdeI and XhoI sites (underlined) were incorporated into the 5’ ends of the CS-F and CS-R, respectively. After purification, the PCR products were ligated into the expression vector pET-30a (Novagen), and transformed into E.coli BL21 (DE3) plysS cells. And then we extract and separate the expressed protein from the E.coli, after that, we carried out them by SDS–PAGE according to Li et al. [75].
mRNA extraction, cDNA synthesis and qRT-PCR
Developmental seeds from three spikes were combined together to extract total RNA from endosperm of CS and CS-n, and cDNA synthesis, qRT-PCR were according to Wang et al. [56]. The primers were: LMW-i-F: TGAAGACCTTCCTCGTCTTTG, LMW-i-R: CTGTGAAATTTGCGCAACG. Gene-specific primers were designed using Primer 5.0 and their specificities were checked by the melting curves of the RT-PCR products. Each qRT-PCR reaction was performed in 20 μl volumes containing 10 μl 2 × SYBR® Premix Ex Taq™ (TaKaRa), 2 μl 50-fold diluted cDNA, 0.4 μl of each gene-specific primer and 7.2 μl ddH2O. PCR conditions were as follows: 95°C for 3 min, 45 cycles of 15 s at 95°C, 57°C for 20s and 72°C for 20s. Three replicates were used for each sample. Reactions were conducted in a CFX96 Real-Time PCR Detection System (Bio-Rad). All data were analyzed with CFX Manager Software (Bio-Rad).
Determination of Glu-A3adeletion in CS by STS-PCR marker
To identify the deletion of Glu-A3a in CS-n, we did the STS-PCR marker of the seven NILs, CS-1Sl/1B, S genome addition line, CB037A, CS, CS-n. We used the marker name of glu-A3a to do PCR as what Wang et al. [24] did before. The primer sets are LA1F: AAACAGAATTATTAAAGCCGG, and SA1R: GGTTGTTGTTGTTGCAG CA. Their PCR cycling conditions were 94°C for 4 min, followed by 35 cycles of 94°C for 35 s, 55°C for 45 s, 72°C for 40 s, and a final extension at 72°C for 8 min.
Development and validation of allele-specific PCR markers for Glu-A3a
To identify the Glu-A3a gene in different genotypes, based on the SNPs we detected in Glu-A3a, we designed the primer named Glu-A3a F: GCAAAGAAGGAAAAGAG GTGG, R: GGTTGTTGTTGTTGCTGCA as the primer to discriminate the gene Glu-A3a from others in CS and CS-n, this also validated in four RILs (CB037A and 1Sl/1B), CS-1Sl/1B, 1Sl genome addition line, 7 NILs of Aroona and 48 varieties. PCR cycling conditions were 94°C for 4 min, followed by 35 cycles of 94°C for 35 s, 60°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 8 min.
Additional files
Abbreviations
- 2D-PAGE:
-
Two-dimensional gel electrophoresis (IEF × SDS-PAGE)
- A-PAGE:
-
Acid polyacrylamide gel electrophoresis
- AS-PCR:
-
Allele specific-polymerase chain reaction
- BAC:
-
Bacterial artificial chromosome
- BIP:
-
Binding protein
- CAAS:
-
Chinese Academy of Agricultural Sciences
- CBB:
-
Coomassie brilliant blue
- DPA:
-
Days post anthesis
- GMP:
-
Glutenin macropolymer
- HMW-GS:
-
High-molecular-weight glutenin subunits
- InDels:
-
Insertions/deletions
- LC-MS/MS:
-
Liquid chromatography-mass spectrometer/mass spectrometer
- LMW-GS:
-
Low-molecular-weight glutenin subunits
- MALDI-TOF–MS:
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MCC:
-
Micro-core collections
- NILs:
-
Near-isogenetic lines
- ORFs:
-
Open reading frame
- PDI:
-
Protein disulfide isomerase
- PCR:
-
Polymerase chain reaction
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- RIL:
-
Recombinant inbred line
- RP-UPLC:
-
Reversed-phase ultra performance liquid chromatography
- Rmax:
-
Extensograph maximum resistance
- RWCN:
-
Raw white Chinese noodle
- SNPs:
-
Single nucleotide polymorphisms
- STS-PCR:
-
Sequence-tagged site polymerase chain reaction
- SDS-PAGE:
-
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- ZSV:
-
Zeleny sedimentation value
References
Shewry PR, Halford NG: Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot. 2002, 53 (370): 947-958. 10.1093/jexbot/53.370.947.
Payne PI: Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Annu Rev Plant Physiol. 1987, 38 (1): 141-153. 10.1146/annurev.pp.38.060187.001041.
Cornish G, Bekes F, Allen H, Martin D: Flour proteins linked to quality traits in an Australian doubled haploid wheat population. Crop Pasture Sci. 2001, 52 (12): 1339-1348. 10.1071/AR01060.
Jackson E, Holt L, Payne P: Characterisation of high molecular weight gliadin and low-molecular-weight glutenin subunits of wheat endosperm by two-dimensional electrophoresis and the chromosomal localisation of their controlling genes. Theor Appl Genet. 1983, 66 (1): 29-37. 10.1007/BF00281844.
Yan Y, Prodanovic S, Mladenov N, Milovanovic M: Studies on genetic control of low-molecular-weight glutenin subunits in wheat endosperm by A-PAGE analysis. Cereal Res Comm. 1999, 27 (3): 251-257.
D'Ovidio R, Masci S: The low-molecular-weight glutenin subunits of wheat gluten. J Cereal Sci. 2004, 39 (3): 321-339. 10.1016/j.jcs.2003.12.002.
Shewry P, Miflin B, LEW EJ, Kasarda D: The preparation and characterization of an aggregated gliadin fraction from wheat. J Exp Bot. 1983, 34 (11): 1403-1410. 10.1093/jxb/34.11.1403.
Lee Y-K, Ciaffi M, Appels R, Morell M: The low-molecular-weight glutenin subunit proteins of primitive wheats. II. The genes from A-genome species. Theor Appl Genet. 1999, 98 (1): 126-134. 10.1007/s001220051049.
Kasarda D, Tao H, Evans P, Adalsteins A, Yuen S: Sequencing of protein from a single spot of a 2-D gel pattern: N-terminal sequence of a major wheat LMW-glutenin subunit. J Exp Bot. 1988, 39 (7): 899-906. 10.1093/jxb/39.7.899.
TAO HP, KASARDA DD: Two-dimensional gel map** and N-terminal sequencing of LMW-glutenin subunits. J Exp Bot. 1989, 40 (9): 1015-1020. 10.1093/jxb/40.9.1015.
Lew EJ-L, Kuzmicky D, Kasarda DD: Characterization of low molecular weight glutenin subunits by reversed-phase high-performance liquid chromatography, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and N-terminal amino acid sequencing. Cereal Chem. 1992, 69: 508-508.
Pitts EG, Rafalski JA, Hedgcoth C: Nucleotide sequence and encoded amino acid sequence of a genomic gene region for a low molecular weight glutenin. Nucleic Acids Res. 1988, 16 (23): 11376-10.1093/nar/16.23.11376.
Cloutier S, Rampitsch C, Penner GA, Lukow OM: Cloning and expression of a LMW-i glutenin gene. J Cereal Sci. 2001, 33 (2): 143-154. 10.1006/jcrs.2000.0359.
Ikeda T, Nagamine T, Fukuoka H, Yano H: Identification of new low-molecular-weight glutenin subunit genes in wheat. Theor Appl Genet. 2002, 104 (4): 680-687. 10.1007/s001220100756.
Gupta R, Shepherd K: Two-step one-dimensional SDS-PAGE analysis of LMW subunits of glutelin. Theor Appl Genet. 1990, 80 (1): 65-74. 10.1007/BF00224017.
Dong L, Zhang X, Liu D, Fan H, Sun J, Zhang Z, Qin H, Li B, Hao S, Li Z, Wang D, Zhang A, Ling H: New insights into the organization, recombination, and functional mechanism of low molecular weight glutenin subunit genes in bread wheat. PLoS One. 2010, 5 (10): e13548-10.1371/journal.pone.0013548.
Zhang X, ** H, Zhang Y, Liu D, Li G, **a X, He Z, Zhang A: Composition and functional analysis of low-molecular-weight glutenin alleles with Aroona near-isogenic lines of bread wheat. BMC Plant Biol. 2012, 12 (1): 243-10.1186/1471-2229-12-243.
Zhang X, Liu D, Zhang J, Jiang W, Luo G, Yang W, Sun J, Tong Y, Cui D, Zhang A: Novel insights into the composition, variation, organization, and expression of the low-molecular-weight glutenin subunit gene family in common wheat. J Exp Bot. 2013, 64 (7): 2027-2040. 10.1093/jxb/ert070.
Peña R, Zarco-Hernandez J, Amaya-Celis A, Mujeeb-Kazi A: Relationships between chromosome 1B-encoded glutenin subunit compositions and bread-making quality characteristics of some durum wheat (Triticum turgidum) cultivars. J Cereal Sci. 1994, 19 (3): 243-249. 10.1006/jcrs.1994.1031.
Ito M, Fushie S, Maruyama-Funatsuki W, Ikeda TM, Nishio Z, Nagasawa K, Tabiki T, Yamauchi H: Effect of allelic variation in three glutenin loci on dough properties and bread-making qualities of winter wheat. Breed Sci. 2011, 61 (3): 281-287. 10.1270/jsbbs.61.281.
Liu L, He ZH, Yan J, Zhang Y, **a XC, Pena RJ: Allelic variation at the Glu-1 and Glu-3 loci, presence of the 1B.1R translocation, and their effects on mixographic properties in Chinese bread wheats. Euphytica. 2005, 142 (3): 197-204. 10.1007/s10681-005-1682-4.
Meng XG, **e F, Shang XW, An LZ: Association between allelic variations at the Glu-3 loci and wheat quality traits with Lanzhou Alkaline Stretched Noodles quality in northwest China spring wheats. Cereal Res Comm. 2007, 35 (1): 109-118. 10.1556/CRC.35.2007.1.13.
** H, Zhang Y, Li G, Mu P, Fan Z, **a X, He Z: Effects of allelic variation of HMW-GS and LMW-GS on mixograph properties and Chinese noodle and steamed bread qualities in a set of Aroona near-isogenic wheat lines. J Cereal Sci. 2013, 57: 146-152. 10.1016/j.jcs.2012.10.011.
Wang L, Li G, Peña RJ, **a X, He Z: Development of STS markers and establishment of multiplex PCR for Glu-A3 alleles in common wheat (Triticum aestivum L.). J Cereal Sci. 2010, 51 (3): 305-312. 10.1016/j.jcs.2010.01.005.
He Z, Yang J, Zhang Y, Quail K, Pena R: Pan bread and dry white Chinese noodle quality in Chinese winter wheats. Euphytica. 2004, 139 (3): 257-267. 10.1007/s10681-004-3283-z.
Wieser H, Kieffer R: Correlations of the amount of gluten protein types to the technological properties of wheat flours determined on a micro-scale. J Cereal Sci. 2001, 34 (1): 19-27. 10.1006/jcrs.2000.0385.
Cassidy B, Dvorak J, Anderson O: The wheat low-molecular-weight glutenin genes: characterization of six new genes and progress in understanding gene family structure. Theor Appl Genet. 1998, 96 (6–7): 743-750. 10.1007/s001220050797.
Johal J, Gianibelli M, Rahman S, Morell M, Gale K: Characterization of low-molecular-weight glutenin genes in Aegilops tauschii. Theor Appl Genet. 2004, 109 (5): 1028-1040. 10.1007/s00122-004-1711-z.
Li X, Wang A, **ao Y, Yan Y, He Z, Appels R, Ma W, Hsam S, Zeller F: Cloning and characterization of a novel low molecular weight glutenin subunit gene at the Glu-A3 locus from wild emmer wheat (Triticum turgidum L. var. dicoccoides). Euphytica. 2008, 159 (1–2): 181-190.
Masci S, D'Ovidio R, Lafiandra D, Kasarda DD: Characterization of a low-molecular-weight glutenin subunit gene from bread wheat and the corresponding protein that represents a major subunit of the glutenin polymer. Plant Physiol. 1998, 118 (4): 1147-1158. 10.1104/pp.118.4.1147.
D’Ovidio R, Marchitelli C, Cardelli LE, Porceddu E: Sequence similarity between allelic Glu-B3 genes related to quality properties of durum wheat. Theor Appl Genet. 1999, 98 (3–4): 455-461. 10.1007/s001220051091.
Gupta RB, Shepherd KW: Genetic Control of LMW Glutenin Subunits In Bread Wheat And Association With Physical Dough Properties. Proceedings of the 3rd International Workshop on Gluten Proteins, Budapest, Hungary, May 9–12. Edited by: Lasztity R, Bekes F. 1987
Huang X, Cloutier S: Molecular characterization and genomic organization of low molecular weight glutenin subunit genes at the Glu-3 loci in hexaploid wheat (Triticum aestivum L.). Theor Appl Genet. 2008, 116 (7): 953-966. 10.1007/s00122-008-0727-1.
Wang K, Gao L, Wang S, Zhang Y, Li X, Zhang M, **e Z, Yan Y, Belgard M, Ma W: Phylogenetic relationship of a new class of LMW-GS genes in the M genome of Aegilops comosa . Theor Appl Genet. 2011, 122 (7): 1411-1425. 10.1007/s00122-011-1541-8.
Wang LH, Zhao XL, He ZH, Ma W, Appels R, Peña RJ, **a XC: Characterization of low-molecular-weight glutenin subunit Glu-B3 genes and development of STS markers in common wheat (Triticum aestivum L.). Theor Appl Genet. 2009, 118: 525-539. 10.1007/s00122-008-0918-9.
Wang S, Li X, Wang K, Wang X, Li S, Zhang Y, Guo G, Hsam SLK, Zeller FJ, Yan Y: Phylogenetic analysis of C, U, N and M genomes and their relationships with Triticum and other related genomes as revealed by LMW-GS genes at Glu-3 loci. Genome. 2011, 54 (4): 273-284. 10.1139/g10-119.
Zhao H, Wang R, Guo A, Hu S, Sun G: Development of primers specific for LMW-GS genes located on chromosome 1D and molecular characterization of a gene from Glu-D3 complex locus in bread wheat. Hereditas. 2004, 141 (3): 193-198. 10.1111/j.1601-5223.2004.01852.x.
Jiang C, Pei Y, Zhang Y, Li X, Yao D, Yan Y, Ma W, Hsam S, Zeller F: Molecular cloning and characterization of four novel LMW glutenin subunit genes from Aegilops longissima, Triticum dicoccoides and T. zhukovskyi . Hereditas. 2008, 145 (2): 92-98. 10.1111/j.0018-0661.2008.02035.x.
An X, Zhang Q, Yan Y, Li Q, Zhang Y, Wang A, Pei Y, Tian J, Wang H, Hsam S: Cloning and molecular characterization of three novel LMW-i glutenin subunit genes from cultivated einkorn (Triticum monococcum L.). Theor Appl Genet. 2006, 113 (3): 383-395. 10.1007/s00122-006-0299-x.
Maruyama Funatsuki W, Takata K, Funatsuki H, Tabiki T, Ito M, Nishio Z, Kato A, Saito K, Yahata E, Saruyama H, Yamauchi H: Identification and characterization of a novel LMW-s glutenin gene of a Canadian Western Extra-Strong wheat. J Cereal Sci. 2005, 41: 47-57. 10.1016/j.jcs.2004.07.003.
Zhang W, Gianibelli MC, Rampling LR, Gale KR: Characterisation and marker development for low molecular weight glutenin genes from Glu-A3 alleles of bread wheat (Triticum aestivum. L). Theor Appl Genet. 2004, 108: 1409-1419. 10.1007/s00122-003-1558-8.
Johal J, Gianibelli MC, Rahman S, Morell MK, Gale KR: Characterization of low-molecular-weight glutenin genes in Aegilops tauschii . Theor Appl Genet. 2004, 109: 1028-1040. 10.1007/s00122-004-1711-z.
Zhao XL, **a XC, He ZH, Lei ZS, Appels R, Yang Y, Sun QX, Ma WJ: Novel DNA variations to characterize low molecular weight glutenin Glu-D3 genes and develop STS markers in common wheat. Theor Appl Genet. 2007, 114: 451-460. 10.1007/s00122-006-0445-5.
Huang Z, Long H, Wei YM, Yan ZH, Zheng YL: Molecular characterization of novel low-molecular-weight glutenin genes in Aegilops longissima . J Appl Genet. 2010, 51 (1): 9-18. 10.1007/BF03195705.
Gupta R, Singh N, Shepherd K: The cumulative effect of allelic variation in LMW and HMW glutenin subunits on dough properties in the progeny of two bread wheats. Theor Appl Genet. 1989, 77 (1): 57-64. 10.1007/BF00292316.
Harberd NP, Bartels D, Thompson RD: Analysis of the gliadin multigene loci in bread wheat using nullisomic-tetrasomic lines. Mol Gene Genet. 1985, 198: 234-242. 10.1007/BF00383001.
Sabelli PA, Shewry PR: Characterization and organization of gene families at the Gli-1 loci of bread and durum wheats by restriction fragment analysis. Theor Appl Genet. 1991, 83: 209-216. 10.1007/BF00226253.
Rasheed A, **a X, Yan Y, Appels R, Mahmood T, He Z: Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J Cereal Sci. 2014, 60: 11-24. 10.1016/j.jcs.2014.01.020.
Masci S, D’ovidio R, Lafiandra D, Kasarda D: A 1B-coded low-molecular-weight glutenin subunit associated with quality in durum wheats shows strong similarity to a subunit present in some bread wheat cultivars. Theor Appl Genet. 2000, 100 (3): 396-400. 10.1007/s001220050052.
Tatham AS, Field JM, Smith SJ, Shewry PR: The conformations of wheat gluten proteins. II. Aggregated gliadins and low molecular weight subunits of glutenin. J Cereal Sci. 1987, 51: 203-214. 10.1016/S0733-5210(87)80023-1.
Tatham AS, Drake AF, Shewry PR: Conformational studies of synthetic peptides corresponding to the repetitive region of the high molecular weight (HMW) glutenin subunits of wheat. J Cereal Sci. 1990, 11: 189-200. 10.1016/S0733-5210(09)80163-X.
Wang K, An X, Pan L, Dong K, Gao L, Wang S, **e Z, Zhang Z, Appels R, Ma W, Yan Y: Molecular characterization of HMW-GS 1Dx3 t and 1Dx4 t genes from Aegilops tauschii and their potential value for wheat quality improvement. Hereditas 2012, 149:41–49.,
Butow B, Ma W, Gale K, Cornish G, Rampling L, Larroque O, Morell M, Békés F: Molecular discrimination of Bx7 alleles demonstrates that a highly expressed high-molecular-weight glutenin allele has a major impact on wheat flour dough strength. Theor Appl Genet. 2003, 107 (8): 1524-1532. 10.1007/s00122-003-1396-8.
Gupta RB, Masci S, Lafiandra D, Bariana HS, MacRitchie F: Accumulation of protein subunits and their polymers in develo** grains of hexaploid wheats. J Exp Bot. 1996, 47 (9): 1377-1385. 10.1093/jxb/47.9.1377.
Liu W, Zhang Y, Gao X, Wang K, Wang S, Zhang Y, He Z, Ma W, Yan Y: Comparative proteome analysis of glutenin synthesis and accumulation in develo** grains between superior and poor quality bread wheat cultivars. J Sci Food Agri. 2012, 92: 106-115. 10.1002/jsfa.4548.
Li J, Han C, Zhen S, Li X, Yan Y: Characterization of HMW glutenin subunit Bx7OE and its distribution in common wheat and related species. Plant Genetic Res: Characterization and Utilization. 2014, 12 (2): 191-198. 10.1017/S1479262113000476.
Dupont F, Altenbach S: Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J Cereal Sci. 2003, 38 (2): 133-146. 10.1016/S0733-5210(03)00030-4.
Wang S, Yu Z, Cao M, Shen X, Li N, Li X, Ma W, Weißgerber H, Zeller F, Hsam S: Molecular mechanisms of HMW glutenin subunits from 1S l genome of Aegilops longissima positively affecting wheat breadmaking quality. PLoS One 2013, 8(4):e58947.,
Li X, Wang K, Wang S, Gao L, **e X, Hsam SLK, Zeller FJ, Yan Y: Molecular characterization and comparative transcript analysis of two subclasses among LMW-m type genes from Aegilops species and bread wheat (Triticum aestivum L.). Theor Appl Genet. 2010, 121: 845-856. 10.1007/s00122-010-1354-1.
Andrews JL, Hay RL, Skerritt JH, Sutton KH: HPLC and immunoassay-based glutenin subunit analysis: screening for dough properties in wheats grown under different environmental conditions. J Cereal Sci. 1994, 20: 203-215. 10.1006/jcrs.1994.1060.
Long H, Wei YM, Yan ZH, Baum B, Nevo E, Zhen YL: Classification of wheat low-molecular-weight glutenin subunit genes and its chromosome assignment by develo** LMW-GS group-specific primers. Theor Appl Genet. 2005, 111: 1251-1259. 10.1007/s00122-005-0024-1.
Ikeda TM, Araki E, Fujita Y, Yano H: Characterization of low molecular-weight glutenin subunit genes and their protein products in common wheats. Theor Appl Genet. 2006, 112: 327-334. 10.1007/s00122-005-0131-z.
Zhao XL, Ma W, Gale KR, Lei ZS, He ZH, Sun QX, **a XC: Identification of SNPs and development of functional markers for LMW-GS genes at Glu-D3 and Glu-B3 loci in bread wheat (Triticum aestivum L.). Mol Breed. 2007, 20: 223-231. 10.1007/s11032-007-9085-y.
Zhao XL, **a XC, He ZH, Gale KR, Lei ZS, Appels R, Ma WJ: Characterization of three low-molecular-weight Glu-D3 subunit genes in common wheat. Theor Appl Genet. 2006, 113: 1247-1259. 10.1007/s00122-006-0379-y.
Wang A, Gao L, Li X, Zhang Y, He Z, **a X, Zhang Y, Yan Y: Characterization of two 1D-encoded ω-gliadin subunits closely related to dough strength and pan bread-making quality in common wheat (Triticum aestivum L.). J Cereal Sci 2008, 47(3):528–535.,
Yan Y, Hsam S, Yu J, Jiang Y, Zeller F: Allelic variation of the HMW glutenin subunits in Aegilops tauschii accessions detected by sodium dodecyl sulphate (SDS-PAGE), acid polyacrylamide gel (A-PAGE) and capillary electrophoresis. Euphytica. 2003, 130 (3): 377-385. 10.1023/A:1023062316439.
Yan Y, Jiang Y, Sun M, Yu J, **ao Y, Zheng J, Hu Y, Cai M, Li Y, Hsam SL: Rapid identification of HMW glutenin subunits from different hexaploid wheat species by acidic capillary electrophoresis. Cereal Chem. 2004, 81 (5): 561-566. 10.1094/CCHEM.2004.81.5.561.
Yan Y, Yu J, Jiang Y, Hu Y, Cai M, Hsam SL, Zeller FJ: Capillary electrophoresis separation of high molecular weight glutenin subunits in bread wheat (Triticum aestivum L.) and related species with phosphate-based buffers. Electrophoresis. 2003, 24 (9): 1429-1436. 10.1002/elps.200390184.
Yu Z, Han C, Yan X, Li X, Jiang G, Yan Y: Rapid characterization of wheat low molecular weight glutenin subunits by ultra performance liquid chromatography (UPLC). J Agric Food Chem. 2013, 61 (17): 4026-4034. 10.1021/jf400472s.
Pei Y, Wang A, An X, Li X, Zhang Y, Huang X, Yan Y: Characterization and comparative analysis of three low molecular weight glutenin C-subunit genes isolated from Aegilops tauschii . Can J Plant Sci. 2007, 87 (2): 273-280. 10.4141/P06-152.
Lv D, Subburaj S, Cao M, Yan X, Li X, Appels R, Sun D, Ma W, Yan Y: Proteome and phosphoproteome reveals new response and defense mechanisms of Brachypodium distachyon leaves under salt stress. Mol Cell Proteomics. 2013, 13 (2): 632-652. 10.1074/mcp.M113.030171.
** M, **e Z-Z, Ge P, Li J, Jiang S-S, Subburaj S, Li X-H, Zeller F-J, Hsam S-L-K, Yan Y-M: Identification and molecular characterisation of HMW glutenin subunit 1By16* in wild emmer. J Appl Genet. 2012, 53 (3): 249-258. 10.1007/s13353-012-0101-5.
Sun H, Yan S, Jiang W, Li G, MacRitchie F: Contribution of lipid to physicochemical properties and Mantou-making quality of wheat flour. Food Chem. 2010, 121 (2): 332-337. 10.1016/j.foodchem.2009.12.033.
McDonald M, Elliot L, Sweeney P: DNA extraction from dry seeds for RAPD analyses in varietal identification studies. Seed Sci Tech. 1994, 22 (1): 171-176.
Li X, Zhang Y, Gao L, Wang A, Ji K, He Z, Appels R, Ma W, Yan Y: Molecular cloning, heterologous expression, and phylogenetic analysis of a novel y-type HMW glutenin subunit gene from the G genome of Triticum timopheevii . Genome. 2007, 50 (12): 1130-1140. 10.1139/G07-089.
Gaut BS, Morton BR, McCaig BC, Clegg MT: Substitution rate comparisons between grasses and palms: synonymous rate differences at the nuclear gene Adh parallel rate differences at the plastid gene rbcL. Proc Natl Acad Sci. 1996, 93 (19): 10274-10279. 10.1073/pnas.93.19.10274.
Kumar S, Tamura K, Nei M: MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinformatics. 2004, 5 (2): 150-163. 10.1093/bib/5.2.150.
Acknowledgements
This research was financially supported by grants from the National Natural Science Foundation of China (31271703, 31471485), International Science & Technology Cooperation Program of China (2013DFG30530), the National Key Project for Transgenic Crops in China (2014ZX08009-003), Natural Science Foundation of Bei**g City and the Key Developmental Project of Science Technology, Bei**g Municipal Commission of Education (KZ201410028031).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZS, HC and MC carried out all experiments and data analysis. GA, SX, ZM and LX performed the preparation of DNA, RNA, cDNA, PCR, qRT-PCR and 2-D. YY conceived the study, planned experiments, and helped draft the manuscript. All authors read and approved the final manuscript.
Caixia Han, Chaoying Ma contributed equally to this work.
Electronic supplementary material
12870_2014_367_MOESM1_ESM.jpeg
Additional file 1: Figure S1.: Plant, Spikelet and seeds morphology of CS and CS-n. A: Plant morphology of CS and CS-n. B1 and B2 are Spikelet morphology of CS and CS-n, respectively. C1 and C2 are the seeds of CS and CS-n, separately. (JPEG 100 KB)
12870_2014_367_MOESM2_ESM.jpeg
Additional file 2: Figure S2.: Kernel morphology of different developmental times of CS and CS-n. The seeds are 5, 8, 11, 14, 17, 20, 23, 26, 29 DPAs from CS and CS-n respectively. The characterization of them is similar. (JPEG 985 KB)
12870_2014_367_MOESM3_ESM.xls
Additional file 3: Table S1.: Comparison of data statistics about the two varieties (CS and CS-n). We statistics plant height, Ear length, stronger spikelet number, kernels per spike, grain weight per spike, Tiller number and thousand kernel weight (TKW) of CS, CS-n. (XLS 16 KB)
12870_2014_367_MOESM4_ESM.jpeg
Additional file 4: Figure S3.: The comparison of the albumins, globulins and prolamins in CS and CS-n. 1 and 2 are the albumins of CS-n and CS, respectively. 3 is the globulins of CS-n, 4 is the globulins of CS. 5 and 6 are the prolamins of CS-n and CS, separately. The difference between them was marked by a black arrow. (JPEG 268 KB)
12870_2014_367_MOESM5_ESM.jpeg
Additional file 5: Figure S4.: Identification of Glu-A3a in LMW-GS deletion lines of CS and CS-n by MALDI-TOF–MS. The LMW-GS Glu-A3a is marked by a black arrow. (JPEG 1 MB)
12870_2014_367_MOESM6_ESM.jpeg
Additional file 6: Figure S5.: Agarose gel electrophoresis separation of amplified products from genomic DNA of CS. With the AS-PCR primer, a single band was cloned in CS. Lane1 and Lane2: PCR amplified products and Lane3: 1 kb DNA marker. (JPEG 103 KB)
12870_2014_367_MOESM7_ESM.jpeg
Additional file 7: Figure S6.: Sequence alignment of STS-PCR marker products of CS, Glu-A3a and its CDS. Glu-A3a is the full sequence, and the Glu-A3a CDS is the coding area. Marker is the band we cloned with the marker named glu-A3a from CS. (JPEG 23 KB)
12870_2014_367_MOESM8_ESM.xls
Additional file 8: Table S2.: Comparison of the mature protein sequences of different LMW-i glutenin subunits. The number and positions of cysteine in different LMW-i glutenin subunits are different and relative conservative. (XLS 16 KB)
12870_2014_367_MOESM9_ESM.jpeg
Additional file 9: Figure S7.: qRT-PCR optimization design: double standard curve and dissolution curve of the gene Glu-A3a. The red standard curve represented Glu-A3a gene and the other blue standard curve represented the reference gene. The dissolution curves of different genes were indicated. (JPEG 691 KB)
12870_2014_367_MOESM10_ESM.xlsx
Additional file 10: Table S3.: Validation of the Glu-A3a allele-specific markers with different wheat varieties, a set of NILs and 4 RILs. The source and the allele that contained were listed in this file. (XLSX 5 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Zhen, S., Han, C., Ma, C. et al. Deletion of the low-molecular-weight glutenin subunit allele Glu-A3a of wheat (Triticum aestivumL.) significantly reduces dough strength and breadmaking quality. BMC Plant Biol 14, 367 (2014). https://doi.org/10.1186/s12870-014-0367-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-014-0367-3