Abstract
Background
Unraveling the intricate and tightly regulated process of adipogenesis, involving coordinated activation of transcription factors and signaling pathways, is essential for addressing obesity and related metabolic disorders. The molecular pathways recruited by mesenchymal stem cells (MSCs) during adipogenesis are also dependent on the different sources of the cells and genetic backgrounds of donors, which contribute to the functional heterogeneity of the stem cells and consequently affect the developmental features and fate of the cells.
Methods
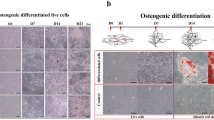
In this study, the alteration of transcripts during differentiation of synovial mesenchymal stem cells (SMSCs) derived from fibrous synovium (FS) and adipose synovial tissue (FP) of two pig breeds differing in growth performance (German Landrace (DL)) and fat deposition (Angeln Saddleback (AS)) was investigated. SMSCs from both tissues and breeds were stimulated to differentiate into adipocytes in vitro and sampled at four time points (day 1, day 4, day 7 and day 14) to obtain transcriptomic data.
Results
We observed numerous signaling pathways related to the cell cycle, cell division, cell migration, or cell proliferation during early stages of adipogenesis. As the differentiation process progresses, cells begin to accumulate intracellular lipid droplets and changes in gene expression patterns in particular of adipocyte-specific markers occur. PI3K-Akt signaling and metabolic pathways changed most during adipogenesis, while p53 signaling and ferroptosis were affected late in adipogenesis. When comparing MSCs from FS and FP, only a limited number of differentially expressed genes (DEGs) and enriched signaling pathways were identified. Metabolic pathways, including fat, energy or amino acid metabolism, were highly enriched in the AS breed SMSCs compared to those of the DL breed, especially at day 7 of adipogenesis, suggesting retention of the characteristic metabolic features of their original source, demonstrating donor memory in culture. In contrast, the DL SMSCs were more enriched in immune signaling pathways.
Conclusions
Our study has provided important insights into the dynamics of adipogenesis and revealed metabolic shifts in SMSCs associated with different cell sources and genetic backgrounds of donors. This emphasises the critical role of metabolic and genetic factors as important indications and criteria for donor stem cell selection.
Similar content being viewed by others
Background
The mesenchymal stem cells (MSCs) are multipotent progenitors cells that can differentiate into a variety of cell types, including bone cells, cartilage cells, and fat cells [1]. Adipogenesis, the differentiation of mesenchymal stem cells into adipocytes, entails activating transcription factors and signaling pathways. It comprises a two-step process: stem cell determination and preadipocyte differentiation [2,3,4]. Briefly, at the beginning of adipogenesis is the signaling of bone morphogenetic proteins (BMPs), which belong to the transforming growth factor β (TGF-β) superfamily, a family of proteins that play a role in the conversion of pluripotent stem cells into the adipocyte lineage [5]. Then it is often portrayed as a cascade of regulatory events, the first wave involving CCAAT/enhancer-binding protein β (C/EBPβ) and C/EBPδ and sterol-regulatory element binding protein 1 (SREBP1), and these transcription factors being involved in the activation of the second wave, which includes C/EBPα and PPARγ, which coordinately activate the transcription of genes that give rise to the adipocyte phenotype [4].
Pigs, due to their physiological similarities to humans, serve as valuable cellular models for advancing stem cell therapies, regenerative medicine, and transplantation [6, 7]. Porcine MSCs are utilized as large animal models in regenerative medicine, preclinical studies, and transplantation for both human and veterinary applications, benefiting the livestock industry [7,8,9]. Comprehensive understanding of molecular changes during MSC transition from self-renewal to differentiation, particularly in pig adipogenesis, and related technologies, contributes to diverse experimental research.
Various source of tissue for derived MSCs influence functional properties of the cells was reported [10,5.
Data availability
The expression datasets for this study can be found in the Gene Expression Omnibus public repository with the GEO accession number (GSE232501: GSM7349177-GSM7349248).
References
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Sci (New York NY). 1999;284(5411):143–7.
Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signaling. Trends Endocrinol Metab. 2009;20(1):16–24.
Fink T, Zachar V. Adipogenic differentiation of human mesenchymal stem cells. Methods Mol Biol. 2011;698:243–51.
MacDougald OA, Mandrup S. Adipogenesis forces that tip thescales. Trends Endrocrinol Metab. 2002;13(1):5–11.
Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40(4):229–42.
Sridharan D, Pracha N, Rana SJ, Ahmed S, Dewani AJ, Alvi SB, Mergaye M, Ahmed U, Khan M. Preclinical large animal Porcine models for Cardiac Regeneration and its clinical translation: role of hiPSC-Derived cardiomyocytes. Cells 2023, 12(7).
Bharti D, Shivakumar S, Subbarao R, Rho G. Research Advancements in Porcine Derived mesenchymal stem cells. Curr Stem Cell Res Ther. 2016;11(1):78–93.
Arrizabalagaa JH, Nollerta MU. Properties of porcine adipose-derived stem cells and their applications in preclinical models. Adipocyte. 2017;6(3):217–23.
Rubessa M, Polkoff K, Bionaz M, Monaco E, Milner DJ, Holllister SJ, Goldwasser MS, Wheeler MB. Use of Pig as a model for mesenchymal stem cell therapies for bone regeneration. Anim Biotechnol. 2017;28(4):275–87.
Mohamed-Ahmed S, Fristad I, Lie S, Suliman S, Mustafa K, Vindenes H, Idris SB. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther 2018, 9(1).
Xu L, Liu Y, Sun Y, Wang B, **ong Y, Wei Q, Wang H, He W, Wang B, Li G. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther. 2017;8(1):275.
Siengdee P, Oster M, Reyer H, Viergutz T, Wimmers K, Ponsuksili S. Morphological and molecular features of porcine mesenchymal stem cells derived from different types of synovial membrane, and genetic background of cell donors. Front Cell Dev Biol. 2020;8:601212.
Rashid U, Yousaf A, Yaqoob M, Saba E, Moaeen-Ud-Din M, Waseem S, Becker SK, Sponder G, Aschenbach JR, Sandhu MA. Characterization and differentiation potential of mesenchymal stem cells isolated from multiple canine adipose tissue sources. BMC Vet Res. 2021;17(1):388.
Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54(3):843–53.
Sasaki A, Mizuno M, Ozeki N, Katano H, Otabe K, Tsuji K, Koga H, Mochizuki M, Sekiya I. Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS ONE. 2018;13(8):e0202922.
Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327(3):449–62.
Tangchitphisut P, Srikaew N, Numhom S, Tangprasittipap A, Woratanarat P, Wongsak S, Kijkunasathian C, Hongeng S, Murray IR, Tawonsawatruk T. Infrapatellar Fat Pad: An Alternative Source of Adipose-Derived Mesenchymal Stem Cells. Arthritis 2016, 2016:4019873.
Katagiri K, Matsukura Y, Muneta T, Ozeki N, Mizuno M, Katano H, Sekiya I. Fibrous synovium releases higher numbers of mesenchymal stem cells than adipose synovium in a suspended Synovium Culture Model. Arthroscopy. 2017;33(4):800–10.
Zhang GH, Lu JX, Chen Y, Zhao YQ, Guo PH, Yang JT, Zang RX. Comparison of the adipogenesis in intramuscular and subcutaneous adipocytes from Bamei and Landrace pigs. Biochem cell Biology = Biochimie et Biol cellulaire. 2014;92(4):259–67.
**ao Y, Zhu Q, Liu X, Jiang M, Hao H, Zhu H, Cowan PJ, He X, Liu Q, Zhou S, et al. High-fat diet selectively decreases bone marrow lin(-) /CD117(+) cell population in aging mice through increased ROS production. J Tissue Eng Regen Med. 2020;14(6):884–92.
Ulum B, Teker HT, Sarikaya A, Balta G, Kuskonmaz B, Uckan-Cetinkaya D, Aerts-Kaya F. Bone marrow mesenchymal stem cell donors with a high body mass index display elevated endoplasmic reticulum stress and are functionally impaired. J Cell Physiol. 2018;233(11):8429–36.
Monaco E, Bionaz M, Rodriguez-Zas S, Hurley WL, Wheeler MB. Transcriptomics comparison between porcine adipose and bone marrow mesenchymal stem cells during in vitro osteogenic and adipogenic differentiation. PLoS ONE. 2012;7(3):e32481.
Xu X, Li X, Yan R, Jiang H, Wang T, Fan L, Wu J, Cao J, Li W. Gene expression profiling of human bone marrow-derived mesenchymal stem cells during adipogenesis. Folia Histochem Cytobiol. 2016;54(1):14–24.
Liang Y, Russell I, Walworth C, Chen C. Gene expression in stem cells. Crit Rev Eukaryot. 2009;19(4):289–300.
Robert AW, Angulski ABB, Spangenberg L, Shigunov P, Pereira IT, Bettes PSL, Naya H, Correa A, Dallagiovanna B, Stimamiglio MA. Gene expression analysis of human adipose tissue-derived stem cells during the initial steps of in vitro osteogenesis. Sci Rep. 2018;8(1):4739.
Sisakhtnezhad S, Alimoradi E, Akrami H. External factors infuencing mesenchymal stem cell fate in vitro. Eur J Cell Biol. 2017;96:13–33.
Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14(11):1293–307.
Ganbold M, Ferdousi F, Arimura T, Tominaga K, Isoda H. New Amphiphilic Squalene Derivative improves metabolism of Adipocytes Differentiated from Diabetic adipose-derived stem cells and prevents excessive lipogenesis. Front Cell Dev Biol. 2020;8:577259.
Imhoff BR, Hansen JM. Differential Redox potential profiles during adipogenesis and osteogenesis. Cell Mol Biol Lett. 2011;16(1):149–61.
Sanz C, Vázquez P, Blázquez C, Barrio PA, Alvarez MDM, Blázquez E. Signaling and biological effects of glucagon-like peptide 1 on the differentiation of mesenchymal stem cells from human bone marrow. Am J Physiol Endocrinol Metabolism. 2009;298(3):E634–643.
Savova MS, Mihaylova LV, Tews D, Wabitsch M, Georgiev MI. Targeting PI3K/AKT signaling pathway in obesity. Biomed Pharmacotherapy = Biomedecine Pharmacotherapie. 2023;159:114244.
Xu J, Liao K. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem. 2004;279(34):35914–22.
Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271(49):31372–8.
Hallenborg P, Fjære E, Liaset B, Petersen RK, Murano I, Sonne SB, Falkerslev M, Winther S, Jensen BA, Ma T, et al. p53 regulates expression of uncoupling protein 1 through binding and repression of PPARγ coactivator-1α. Am J Physiol Endocrinol Metabolism. 2016;310(2):E116–128.
Molchadsky A, Ezra O, Amendola PG, Krantz D, Kogan-Sakin I, Buganim Y, Rivlin N, Goldfinger N, Folgiero V, Falcioni R, et al. p53 is required for brown adipogenic differentiation and has a protective role against diet-induced obesity. Cell Death Differ. 2013;20(5):774–83.
Krstic J, Reinisch I, Schupp M, Schulz TJ, Prokesch A. p53 functions in adipose tissue metabolism and homeostasis. Int J Mol Sci 2018, 19(9).
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al. Ferroptosis: a regulated cell death Nexus linking metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273–85.
Hatakeyama A, Uchida S, Utsunomiya H, Tsukamoto M, Nakashima H, Nakamura E, Pascual-Garrido C, Sekiya I, Sakai A. Isolation and characterization of synovial mesenchymal stem cell derived from hip joints: a comparative analysis with a matched control knee Group. Stem Cells Int. 2017;2017:9312329.
Nakashima H, Uchida S, Hatakeyama A, Murata Y, Yamanaka Y, Tsukamoto M, Sekiya I, Sakai A. Isolation and characterization of synovial mesenchymal stem cells derived from patients with chronic lateral ankle instability: a comparative analysis of synovial fluid, adipose synovium, and fibrous synovium of the Ankle Joint. Orthop J Sports Med. 2022;10(5):23259671221094615.
Murata Y, Uchida S, Utsunomiya H, Hatakeyama A, Nakashima H, Chang A, Sekiya I, Sakai A. Synovial mesenchymal stem cells derived from the Cotyloid Fossa Synovium have higher self-renewal and differentiation potential than those from the Paralabral Synovium in the Hip Joint. Am J Sports Med. 2018;46(12):2942–53.
Tencerova M, Figeac F, Ditzel N, Taipaleenmäki H, Nielsen TK, Kassem M. High-Fat Diet-Induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J bone Mineral Research: Official J Am Soc Bone Mineral Res. 2018;33(6):1154–65.
Zong Q, Bundkirchen K, Neunaber C, Noack S. Are the properties of Bone Marrow-derived mesenchymal stem cells influenced by overweight and obesity? Int J Mol Sci 2023, 24(5).
Boland L, Bitterlich LM, Hogan AE, Ankrum JA, English K. Translating MSC Therapy in the age of obesity. Front Immunol. 2022;13:943333.
Wang T, Zhang J, Liao J, Zhang F, Zhou G. Donor genetic backgrounds contribute to the functional heterogeneity of stem cells and clinical outcomes. Stem Cells Translational Med. 2020;9(12):1495–9.
Giralt M. F Villarroya 2013 White, brown, beige/brite: different adipose cells for different functions? Endocrinology 154 9 2992–3000.
Levental KR, Surma MA, Skinkle AD, Lorent JH, Zhou Y, Klose C, Chang JT, Hancock JF, Levental I. ω-3 polyunsaturated fatty acids direct differentiation of the membrane phenotype in mesenchymal stem cells to potentiate osteogenesis. Sci Adv. 2017;3(11):eaao1193.
Chui PC, Guan HP, Lehrke M, Lazer MA. PPARγ regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J Clin Invest. 2005;115(8):2244–56.
Huang B, Hai DY, Do YK, Hai YQ, Sung HC. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-γ (PPARγ) and AMP-activated protein kinase (AMPK) pathways. J Agric Food Chem. 2011;59(8):3666–73.
Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16(1):22–6.
Yu WH, Li FG, Chen XY, Li JT, Wu YH, Huang LH, Wang Z, Li P, Wang T, Lahn BT, et al. PPARγ suppression inhibits adipogenesis but does not promote osteogenesis of human mesenchymal stem cells. Int J Biochem Cell Biol. 2012;44(2):377–84.
Saidova AA, Vorobjev IA. Lineage commitment, signaling pathways, and the cytoskeleton systems in mesenchymal stem cells. Tissue Eng Part B Rev 2020, 26(1).
Tatu CS, Bo** FM, Alexandra GT, Ordodi VL, Mic FA, Vlad I, Cean A, Gavriliuc OI, Paunescu V. Features of lipid metabolism along differentiation pathway of human mesenchymal stem cells towards mature adipocytes. Rom Biotechnol Lett. 2014;19(2):9257–71.
Ponsuksili S, Murani E, Hadlich F, Iqbal MA, Fuchs B, Galuska CE, Perdomo-Sabogal A, Sarais F, Trakooljul N, Reyer H, et al. Prenatal transcript levels and metabolomics analyses reveal metabolic changes associated with intrauterine growth restriction and sex. Open Biol. 2022;12(9):220151.
Acknowledgements
We would like to express special thanks to Joana Bittner, Nicole Gentz, and Annette Jugert for their commitment and excellent technical assistance.
Funding
The Research Institute for Farm Animal Biology (FBN) provided own matched funding. The publication of this article was funded by the Open Access Fund of the Research Institute for Farm Animal Biology (FBN). Open Access funding enabled and organized by Projekt DEAL.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Performed data analysis, data curation, writing original draft, S.P.; Conceived and designed P.S., and S. P.; Conceptualization, supervision, help analysis data, S.L and S.P.; writing, review & editing, W.K., M.O., H.R., and K.W.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is reported in accordance with ARRIVE guidelines. The animals were obtained from the experimental pig unit of the Research Institute for Farm Animal Biology, Dummerstorf, Germany and were not part of an animal experiment. Animal husbandry and slaughter followed the guidelines set by the Animal Care Committee the State of Mecklenburg-Western Pomerania, Germany, based on the German Law of Animal Protection. The slaughterhouse is approved by the European Union and the German quality management system QS (MV21212). The handling and killing of the animals were carried out in accordance with applicable laws, relevant guidelines and ethical regulations, and with veterinary inspection certifying the health of the animals.
Consent for publication
Not applicable.
Competing interests
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ponsuksili, S., Siengdee, P., Li, S. et al. Effect of metabolically divergent pig breeds and tissues on mesenchymal stem cell expression patterns during adipogenesis. BMC Genomics 25, 407 (2024). https://doi.org/10.1186/s12864-024-10308-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10308-z