Abstract
Background
High temperatures cause a suite of problems for cells, including protein unfolding and aggregation; increased membrane fluidity; and changes in DNA supercoiling, RNA stability, transcription and translation. Consequently, enhanced thermotolerance can evolve through an unknown number of genetic mechanisms even in the simple model bacterium Escherichia coli. To date, each E. coli study exploring this question resulted in a different set of mutations. To understand the changes that can arise when an organism evolves to grow at higher temperatures, we sequenced and analyzed two previously described E. coli strains, BM28 and BM28 ΔlysU, that have been laboratory adapted to the highest E. coli growth temperature reported to date.
Results
We found three large deletions in the BM28 and BM28 ΔlysU strains of 123, 15 and 8.5 kb in length and an expansion of IS10 elements. We found that BM28 and BM28 ΔlysU have considerably different genomes, suggesting that the BM28 culture that gave rise to BM28 and BM28 ΔlysU was a mixed population of genetically different cells. Consistent with published findings of high GroESL expression in BM28, we found that BM28 inexplicitly carries the groESL bearing plasmid pOF39 that was maintained simply by high-temperature selection pressure. We identified over 200 smaller insertions, deletions, single nucleotide polymorphisms and other mutations, including changes in master regulators such as the RNA polymerase and the transcriptional termination factor Rho. Importantly, this genome analysis demonstrates that the commonly cited findings that LysU plays a crucial role in thermotolerance and that GroESL hyper-expression is brought about by chromosomal mutations are based on a previous misinterpretation of the genotype of BM28.
Conclusions
This whole-genome sequencing study describes genetically distinct mechanisms of thermotolerance evolution from those found in other heat-evolved E. coli strains. Studying adaptive laboratory evolution to heat in simple model organisms is important in the context of climate change. It is important to better understand genetic mechanisms of enhancing thermotolerance in bacteria and other organisms, both in terms of optimizing laboratory evolution methods for various organisms and in terms of potential genetic engineering of organisms most at risk or most important to our societies and ecosystems.
Similar content being viewed by others
Background
With climate change many organisms will have to evolve to survive increasingly warmer environmental niches. It is important for us to understand the nature of these adaptations so that we can monitor ecosystem health, and, in some cases, such as food crops, possibly intervene to accelerate adaptation. Temperature affects the functioning and integrity of important biomolecules in a cell, including DNA, RNA, proteins and lipids. Thus, temperatures around the minimum or maximum growth temperatures (Tmin or Tmax) of an organism present many problems, requiring the induction of cold shock or heat shock responses for organism survival. High temperatures can unfold or misfold proteins, mRNAs and structural RNAs; lead to aggregation of proteins due to unfolding or misfolding; cause increased membrane fluidity and permeability and cause changes in DNA topology and genome structure. To compensate for these effects, heat-loving organisms, or thermophiles, have more stable proteins and structural RNAs [1, 2], different membrane compositions [3] (e.g., more saturated, long chain and branched chain fatty acids [4]) and other variations compared to moderate-temperature-loving mesophiles or cold-loving psychrophiles. Thermophilic proteins can have enhanced core packing [5], more charged and hydrophobic residues and less polar residues [5,6,7], and more residues involved in secondary structure elements [7] and other interactions such as salt bridges [5, 7, 8].
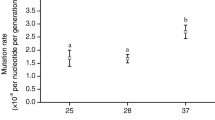
Several Escherichia coli studies [9,10,11,12,13] have pushed the cells towards higher growth temperatures, successfully increasing the natural Tmax of ~45.5 °C in rich liquid medium, ~46.5 °C on rich solid medium or ~43–44 °C in liquid media without methionine by up to 3 °C. These impressive feats were accomplished by adaptive laboratory evolution (ALE) to heat, where the cells were grown at progressively higher temperatures, often with simultaneous hyper-mutagenesis. In our interpretation of the literature, the earliest of these studies resulted in the highest Tmax reported to date [9]. In the study, Rudolph et al. sequenced select heat shock genes and their promoter regions but whole-genome sequencing (WGS) of the heat-evolved strain was not performed. In the 2012 study by Blaby et al. [10], they found a deletion of the glycerol transporter glpF and showed that deletion of glpF significantly enhanced thermotolerance in the MG1655 wildtype background, and they found that a fabA fatty acid desaturase/isomerase mutation increased the amount of saturated and decreased the amount of unsaturated fatty acids in the membrane. However, other mutations in the strain are probably important for the enhanced thermotolerance, including perhaps the mutations in the bifunctional (p)ppGpp synthase/hydrolase spoT and the housekee** σ factor rpoD. In a study by Luan et al. published in 2015 [11], the authors found hundreds of changes compared to the DH5α starting strain, including mutations in the alternate σ factor rpoS and the transcriptional repressor cytR. For the two remaining studies [12, 13], researchers found mutations in spoT for all strains or populations, and changes in the RNA polymerase beta’ subunit rpoC, rpoD, the transcriptional terminator rho and in or around the chaperone groESL in some strains. In less related studies in minimal media, E. coli strains were adapted to constant temperatures between the optimum growth temperature (Topt, 37 °C) and the Tmax (~43–44 °C) growth temperature, at 42 or 42.2 °C [BM28 and BM28 ΔlysU have three large chromosomal deletions We aligned the MG1655, BM28 and BM28 ΔlysU genomes with Mauve [23] and visualized the level of identity (Fig. 1). Compared to MG1655, BM28 and BM28 ΔlysU have three large deletions approximately 123 kb, 15 kb and 8.5 kb in length (Fig. 1 and Supplementary Table S3). The 123 kb deletion spans MG1655 genomic positions 0.251–0.374 Mb, beginning within the dinB gene, ending within the mhpE gene, and fully deleting the 132 genes between them. This deletion creates a gene encoding a DinB-MhpE fusion protein, including amino acids 1–152 of DinB and residues 80–337 of MhpE. Interestingly, residues 149–152 of DinB and 80–83 of MhpE are identical (AKIA) and the two genes share 12 bp of identical DNA sequence in this region (Supplementary Fig. S1). Thus, we inferred that this deletion resulted from homologous recombination between these 12 bp of identical sequence, deleting 123 kb in the process. Mauve genome alignment [23] of the MG1655, BM28 and BM28 ΔlysU genomes showing the level of identity. The numbering corresponds to the MG1655 genome. There are three gaps in identity that correspond to three large deletions in BM28 and BM28 ΔlysU, of lengths 123 kb (from dinB to mhpE), 15 kb (the e14 prophage) and 8.5 kb (from wbbL to rfbD) The 15 kb deletion represents a precise deletion of the cryptic prophage e14 which contains 24 genes (Fig. 1 and Supplementary Table S3). This prophage exists at 1.197–1.211 Mb in MG1655, is flanked by host genes icd and icdC and is excised upon induction of the SOS (DNA damage) response. In a study demonstrating that cryptic prophages contribute to resistance to various stressors [24], Wang et al. showed that in the K-12 strain BW25113, deletion of the e14 prophage did not reduce the cells’ viability to heat shock (at 65 °C for 10 min). As BM28 is a K-12 (MG1655) derivative, the e14 prophage may also not be important for high temperature growth and/or survival in BM28 strains nor their ancestors. The other large deletions and insertion sequence transposition events discussed later likely caused the excision of the e14 prophage, through triggering the SOS response. Finally, the 8.5 kb deletion from 2.103–2.111 Mb in MG1655 occurs within a cluster of O-antigen synthesis genes, deleting eight genes and partially deleting the IS5-interrupted wbbL and uninterrupted rfbD genes (Fig. 1 and Supplementary Table S3). Due to the interruption of wbbL in MG1655, BM28 and BM28 ΔlysU do not produce an O-antigen and instead only produce the lipid A and core regions of lipopolysaccharide [25]. The exact site of the 8.5 kb deletion and reclosing of the chromosome is between the first base of an insertion sequence element IS5 and a base of rfbD (Supplementary Fig. S2). Thus, it is possible that an additional IS5 element in the same orientation as the one in wbbL was inserted into rfbD, and then the 8.5 kb of DNA was deleted through homologous recombination between the two IS5 elements. Compared to MG1655 we found 233 changes in BM28 and 244 changes in BM28 ΔlysU, and compared to the inferred parent strain JB41 we found 231 and 242 changes in the strains, respectively (Fig. 2 and Supplementary Table S4). Thus, a majority of the remaining 231 or 242 changes in the strains likely arose throughout the heat evolution process. Dividing these numbers of changes by the length of the experiment (1,256 cultures subcultured every 48 h, thus, 2,512 days) results in 0.092 changes per day for BM28 and 0.096 changes per day for BM28 ΔlysU (Supplementary Table S4). Focussing in on the 151 BM28 SNPs and the 164 BM28 ΔlysU SNPs, mutational spectra were constructed (Supplementary Fig. S3). The most common base pair change, C-G —> T-A (a combination of C —> T and G —> A SNPs), accounted for 66% of all SNPs in BM28 and 62% of all SNPs in BM28 ΔlysU. Comparison of the BM28 and BM28 ΔlysU genomes revealed 75 differences, including the expected lysU::cat element for BM28 ΔlysU (Fig. 2 and Supplementary Fig. S4). Compared to MG1655 or JB41, BM28 has 32 of these differences and BM28 ΔlysU has 43 of them. Based on differences between the BM28 genome and the MC4100 lysU::cat genome used to delete ΔlysU from BM28 ΔlysU, it appears that about 55 kb (42–67 kb) of the MC4100 lysU::cat genome was swapped into the BM28 ΔlysU genome, including at least the region spanning the genes yjdN and dcuA (see Additional File S2 for more information). This P1 transduction event accounts for 6 of the 75 differences between BM28 and BM28 ΔlysU, adding 2 changes from the MC4100 lysU::cat genome and presumably removing 4 changes present in BM28 (Additional File S2). Excluding the variations coming from the MC4100 lysU::cat genome, there are 41 BM28 ΔlysU specific changes and 32 BM28 specific changes, for a total of 73 differences between BM28 and BM28 ΔlysU that arose over their evolution to high temperature. This leads us to believe that BM28 stocks were a population of genetically different cells, and thus the isolate chosen by Rudolph et al. for lysU deletion was genetically different than the BM28 isolate that we chose. Since the Winter group diluted their high temperature cultures 1:8 every 48 h, we think it is likely that the culture contained genetically distinct cells. With the average mutation rates (Supplementary Table S4; 0.092 changes per day for BM28 and 0.096 changes per day for BM28 ΔlysU) and numbers of strain specific mutations (32 BM28 specific changes and 41 BM28 ΔlysU specific changes) we estimate that the strains evolved separately for approximately the last year of the experiment (the calculation results in 348 days for BM28 and 426 days for BM28 ΔlysU). While these two strains were isolated from single colonies it is likely that a diversity of distinct strains co-evolved in the cultures. The changes in BM28 and BM28 ΔlysU compared to the reconstructed JB41 parent genome. The outer ring shows changes larger than 6 bp and the inner ring shows changes 6 bp or smaller. BM28 changes are shown as blue arrowheads, BM28 ΔlysU changes are shown as yellow arrowheads, and shared changes are shown as green arrowheads. Generated with Proksee [26] A variety of studies resulted in conflicting conclusions as to whether stressful growth conditions elevate the rate of spontaneous mutations. Rudolph et al. used stress-inducing stationary phase mutagenesis throughout the heat adaptation process, subculturing the cells every 48 h for 1,256 cultures (2,512 days). On average, excluding any changes that were introduced by the lysU deletion, this generated 0.092 and 0.096 changes in each 24-h period for BM28 and BM28 ΔlysU, respectively (Supplementary Table S4). Escherichia coli have spontaneous mutation rates of 10–9-10–10 changes per bp per generation [27], or 0.0046–0.00046 changes per genome per generation. Since the cultures were diluted 1:8, we can assume that the cells doubled approximately three times in the 48-h period, which is equal to 1.5 doublings per day. Assuming this and assuming a high spontaneous mutation rate of 10–9 changes per bp per generation, we would expect 0.0069 changes in every 24-h period. Based on this estimate, it appears that the BM28 strains were hyper-mutagenized over the course of their adaptation to heat, with estimated mutation rates ~ 13 X higher than would be expected based on our rough approximation. In addition to the large BM28 SPAdes scaffold we found that corresponds to the chromosome, SPAdes built one more linear scaffold that was > 500 bp. This 4,875 bp scaffold aligned to various ColE1 family plasmids, containing the ColE1 ori, β-lactamase and a truncated chloramphenicol acetyltransferase gene. The scaffold had over 99% pairwise identity with pBR325 but did not appear to be a complete plasmid sequence. Oddities were also seen in the BM28 chromosome WGS data, such as a 2,065 bp region of high coverage spanning the groESL region which was surrounded by low frequency variations. Further exploration revealed that the missing part of the partial ColE1 plasmid sequence was the E. coli groESL region, and that the low frequency variations seen surrounding the chromosomal groESL region corresponded to sequences surrounding groESL in the plasmid (Fig. 3). We determined that this groESL plasmid was pOF39, generated by Fayet et al. [28], and we submitted the sequence to Genbank (Genbank OP156992.1). Map of the groESL plasmid pOF39, generated with Proksee [26]. Genes or gene fragments are shown in pink, regulatory elements in green, the ori in blue and the T- > C SNP in purple. This plasmid is found in BM28 and not in BM28 ΔlysU In tracking down BM28, we were warned that the thermotolerant phenotype had been known to revert. Thus, we grew BM28 and BM28 ΔlysU at or above 42 °C to prepare glycerol stocks (46.8 °C) and for genomic DNA (gDNA) extractions (42–45 °C). From these glycerol stocks, we found that BM28 isolates carry pOF39 whereas BM28 ΔlysU isolates do not (Supplementary Fig. S5), and we also found no evidence of pOF39 in the BM28 ΔlysU Illumina WGS data. Chemical transformation of DH10B with BM28 gDNA preps yielded carbenicillin-resistant colonies (Supplementary Fig. S6) and a PCR of BM28 gDNA with pOF39 primers produced a product of the expected size (Supplementary Fig. S7). We found a single T to C SNP in the pOF39 sequence, within the discriminator regions of the overlap** σ32 and σ70 groESL promoters (Additional File S2). Discriminators lie between the -10 motifs and the transcription start sites of promoters and some of the bases make contacts with the 1.2 regions of σ factors. This SNP slightly decreases the predicted transcription initiation rate of the σ70 promoter of groESL (from 1030 to 1003 au) by the De Novo DNA Promoter Calculator [29]. Consistent with the Rudolph et al. results, we found no mutation in the chromosomal groESL region of BM28. As well, the primers they used to look for mutations in the groESL region would not bind to the pOF39 groESL region, illustrating no discrepancies between ours and their results. To explore the contribution of the groESL plasmid to the thermotolerance of BM28-related cells, we transformed pOF39 into BM28 ΔlysU and MG1655 and we cured some BM28 of pOF39 with sodium dodecyl sulphate (named BM28c for BM28 cured). We then carried out high temperature liquid growth experiments in the aforementioned strains with and without pOF39, at 46.3 °C and 47.8 °C (Fig. 4). At the lower temperature, all strains with pOF39 grow to significantly higher optical densities than the strains without the plasmid. At the higher temperature, only BM28 (with pOF39) grows to a significantly higher optical density compared to BM28 without pOF39 (BM28c). It should be noted that an increase in optical density does not necessarily indicate growth at that temperature. For example, the cells may grow at the very beginning of the experiment before the liquid media has reached the incubator temperature, and any inclusion body formation in the cells would also increase the optical density of the cultures [30]. Furthermore, the widely reported and accepted Tmax of MG1655 in rich media grown aerobically is 45.5 °C, lower than both temperatures used in this experiment, and yet we still see an increase in the optical density over the course of the experiment. However, likely the same phenomenon has been described by the Van Impe group in a number of liquid culture studies [31,32,33], where they show a thermoresistant fraction of a population of MG1655 growing above their liquid Tmax of 45.5 °C. Final optical densities of MG1655, BM28 ΔlysU and BM28c (BM28 cured of pOF39) with and without pOF39 grown at high temperatures. Cultures were incubated at the indicated temperatures in a shaking water bath for 23 h, in quintuplicate, and their final optical densities were measured and recorded. Statistical comparisons between final optical densities of cells with and without pOF39 were determined using an unpaired t test with Welch correction, and P values > 0.05 are indicated with ns, ≤ 0.001 are indicated with ***, and ≤ 0.0001 are indicated with ****. Generated with GraphPad Prism 9.4.1 We also performed high temperature agar plate growth experiments in the strains with and without pOF39. On agar plates, there were larger differences between MG1655 and the BM28 strains in terms of growth at high temperature. On plates without antibiotics at 46.9–47.0 °C and 47.2–47.3 °C, pOF39 improved growth of all three strains (Supplementary Table S5). Importantly, pOF39 alone increased the Tmax of MG1655 on an agar plate by at least 0.4 °C. In line with this, Rudolph et al. showed that a different groESL plasmid increased the Tmax of their 37 °C-evolved strain in liquid media. On the other hand, BM28 and BM28 ΔlysU showed true growth at both high temperatures even without pOF39. This indicates to us that pOF39 is not the sole contributor to their increased thermotolerance, and that some of the chromosomal changes in the BM28 strains are critical to this phenotype. As mentioned earlier, the BM28 strains carry the Tn10 transposon. Tn10 is a composite transposon flanked by two IS10s on either end; IS10L on the left side (further from tetR) and IS10R on the right side (closer to tetR) [34]. They differ by 19 bases, resulting in IS10L having very low activity due to changes in the promoter region. Analysis of the IS10s on the left and right side of the Tn10 in BM28 and BM28 ΔlysU revealed that the elements on the left were IS10R and the elements on the right were mutated IS10L/IS10R hybrids. Interestingly, the IS10L/R hybrids differ between BM28 and BM28 ΔlysU by four SNPs and small substitutions. In BM28, the IS10L/R element has essentially the IS10L promoter (Supplementary Fig. S8), whereas in BM28 ΔlysU the promoter is essentially IS10R for the first two thirds and IS10L for the last third (Supplementary Fig. S9). The last difference is in the IS10 transposase ORF: BM28 have a V301I mutation in their IS10L/R hybrid but BM28 ΔlysU lack this mutation. Aside from that difference, the IS10L/R hybrid ORFs are IS10L for the first third and IS10R for the last two thirds (Supplementary Figs. S8 and S9). Remarkably, the BM28 genome contains a total of 17 IS10s, with 15 isolated IS10R copies (not part of the Tn10 transposon) inserted throughout the genome (Fig. 5). BM28 ΔlysU contains only 14 isolated IS10Rs, lacking the fliZ-interrupting insertion (Table 1 and Supplementary Fig. S4). IS10 elements operate via a cut-and-paste mechanism and generate a duplication of the 9 bp target sequence upon insertion, forming 9 bp direct repeats flanking themselves. For the 15 isolated BM28 IS10Rs, we analyzed the 9 bp target sequences they inserted into to ascertain the consensus sequence YRCTNNRNN, consistent with previous studies reporting the consensus sequence of the middle seven bases as GCTNAGC (Supplementary Fig. S10) [35]. Except for two elements in each strain, these IS10s are identical to the IS10R elements located in the left positions of the strains’ Tn10 transposons. The two nonidentical IS10s (interrupting fimE and inserting in between mcrB and symE) have a single A —> G silent mutation at nucleotide position 333 of the transposase gene, and they are relatively close to each other in the genome (38 kb apart). Based on their proximity and shared SNP, it is possible that one of these insertions gave rise to the other. IS10R elements and the Tn10 transposon in the BM28 chromosome. A BM28 chromosome map with the positions of the IS10R elements and Tn10 transposon is shown, generated with Proksee [26]. The regions oriC and terC are also displayed. Compared to BM28, BM28 ΔlysU lacks the fliZ IS10R insertion In addition to IS10 expansion, two endogenous insertion sequences were deleted in BM28 and BM28 ΔlysU: insH21 between -ychE- > and -oppA- > and insAB5 between < -flhD- and -uspC- > (Supplementary Table S6). Two small (~200 bp) repeat region deletions were found in BM28, likely arising due to homologous recombination between short repeats (Supplementary Table S6). Comparatively, BM28 ΔlysU lacks the repeat region deletion between fre and fadA (Supplementary Table S6 and Supplementary Fig. S4). Finally, a nearly 1300 bp deletion removed parts of the genes ybfL and ybfD, creating an in-frame YbfL-YbfD fusion protein-encoding gene (Supplementary Table S6). Since BM28 ΔlysU lacks the IS10 insertion in fliZ and the fre/fadA deletion, BM28 likely acquired these changes after the two strains diverged from a common ancestor (approximately 1 year prior to the end of the experiment). Building on this assumption, we can identify the other mutations specific to only one of the two strains as also occurring near the end of their evolutionary pathway (Additional File S2). To further explore BM28 and BM28 ΔlysU mutations, we analyzed the 43 BM28 and 41 BM28 ΔlysU genes frame shifted and/or truncated by SNPs and other small changes (Table 1). Amongst these knocked out genes, we found one putative (yidP) and four or five confirmed (cytR, gadW, fliZ, nimR and slyA, but fliZ is only interrupted in BM28) transcription factors in both (excluding the strains’ glpR interruptions because that gene is already interrupted in MG1655). We next decided to look at classes of genes mutated in the BM28 strains more broadly. Using two E. coli essential gene studies, the Keio collection by the Wanner and Mori groups [37, 38] and the TraDIS study by Goodall et al. [39], we found that both strains have mutations in 15 essential genes, BM28 have intergenic mutations beside three essential genes and BM28 ΔlysU have intergenic mutations beside four essential genes (Table 2). In six of the ten essential genes with amino acid substitutions, these substitutions are predicted to increase the stability of the protein by DDGun [40] (Table 2). The remaining essential gene amino acid substitutions are either predicted to be neutral (two) or destabilizing (two). Though not technically/individually considered essential because there are seven copies of each gene, BM28 strains also have mutations in ribosomal RNA genes (Additional File S2). Using the list of heat shock genes determined by Nonaka et al. [41], we identified mutations in or around three heat shock genes for BM28 and two for BM28 ΔlysU (Table 3). Lastly, we performed Gene Ontology and PANTHER analyses [43,44,45] to determine whether specific genes or intergenic regions near specific genes were more or less often mutated than expected (Supplementary Tables S7 and S8). When we included all genes with changes and genes surrounding intergenic regions with changes, including those that were part of deletions, there were no statistically significant results, indicating that gene regions associated with particular biological processes, molecular functions, cellular components, pathways, etc. were not enriched or de-enriched in mutations in BM28 nor BM28 ΔlysU. However, if we excluded the genes that were completely deleted, we saw an overrepresentation of changes in or surrounding genes with the cellular components membrane (GO:0,016,020) and cellular anatomical entity (GO:0,110,165), and for BM28 ΔlysU only, integral component of membrane (GO:0,016,021) (Supplementary Tables S7 and S8). For both strains we also found an underrepresentation of genes with the cellular component unclassified (UNCLASSIFIED) with intragenic or nearby intergenic changes (Supplementary Tables S7 and S8). Thus, excluding completely deleted genes, genes encoding membrane-associated proteins and proteins that are part of something larger than a protein complex are enriched in intragenic and intergenic changes, whereas genes encoding proteins with unclassified cellular components are de-enriched in intragenic and intergenic changes. SNPs and small indels in and around genes which were neither essential nor heat-induced are listed in Additional File S2 alongside all of the genetic changes in the strains. One such mutation to note present in both strains changed the start codon of flgA from AUG to AUA, which when used as a start codon for GFP decreases the predicted translation rate by over 100 X [46]. The codon 3’ to the wildtype AUG is a CUG, which could also serve as a start codon, but CUG is comparable to AUA in terms of its translation initiation rate in the same GFP study. FlgA helps assemble the P-ring of flagella and its deletion renders cells nonmotile. Indeed, a soft agar motility assay showed that unlike DH10B and MG1655, BM28 and BM28 ΔlysU are nonmotile (Supplementary Table S9).BM28 and BM28 ΔlysU likely evolved separately for the last year of the experiment
Mutation rates from stationary phase culturing of BM28 and BM28 ΔlysU
BM28 cells carry the groESL plasmid pOF39 but BM28 ΔlysU do not
pOF39 contributes to thermotolerance in BM28-related cells
Expansion of IS10 elements, deletion of endogenous insertion sequences and small indels
BM28 and BM28 ΔlysU gene knockouts and mutations in essential and heat shock genes
Discussion
Potential mechanism for the large chromosomal deletion
One surprising finding was the 123 kb deletion that fused dinB and mhpE (Fig. 1 and Supplementary Table S3). Several insertion sequences reside in the deleted region, and there were clearly insertion sequence transposition events happening throughout the strains’ evolution to heat. In particular, IS10 transposition events cause double-stranded breaks through their cut-and-paste or nonreplicative transposition mechanism. In response to a double-stranded break in the chromosome, E. coli have an interesting alternate end joining DNA repair mechanism that relies on (sometimes excessive) DNA resection and ligation to close the chromosome at microhomologous sites [47]. dinB and mhpE share 12 bp of identical sequence (Supplementary Fig. S1), which appear to have served as microhomologous sites to close the chromosome. Whether through insertion sequence transposition(s) or other means, it is likely that a double-stranded break occurred in the 123 kb deleted region and in response, the chromosome was repaired via the alternate end joining mechanism.
Possible sources of plasmid pOF39
The discovery of the presence of plasmid pOF39 begs the question: when and how was it introduced? One possibility is that the plasmid was introduced into the lineage between the BM16 and BM25 isolates, that were evolved at 45 °C and 48 °C, respectively. This ideas is consistent with the 2D gel data, the comment by the authors that BM16 and later isolates fail to grow above 45 °C after high dilutions and the difficulty the BM16 lineage had in making the transition from growth at 45 °C to growth at 47 °C. Plasmid pOF39 was not selected for in the typical and stringent manner of antibiotic selection, but was instead likely maintained because it was beneficial for growth at high temperature. Therefore, it may be that high dilutions lead to complete loss of the plasmid, preventing cell growth at higher temperatures in some lineages. Importantly, Rudolph et al. showed that after a heat shock at 49 °C for 3 h, BM25 shows ~ 5000 X higher viability than BM16. This could be in large part due to the acquisition of pOF39. Alternatively, it is possible that the starting strain contained the pOF39 plasmid, and that a combination of genetic changes and selective pressure at higher incubation temperatures lead to a large increase in GroESL expression levels between isolates BM16 and BM25. The attractiveness of this idea is that it does not require an explanation of how plasmid pOF39 was taken up by E. coli, which is not naturally transformable.
The differences between BM28 and BM28 ΔlysU can be explained by the loss of pOF39
Rudolph et al. observed differences in GroESL levels and viability to heat shock after deletion of lysU, which they attributed to loss of lysU. However, we postulate that these differences are caused by the presence of pOF39 in BM28 and the absence of pOF39 in BM28 ΔlysU. We suspect that the strain that went on to become BM28 ΔlysU was cured of its pOF39 during the P1 transduction process to delete lysU. Taking into consideration the BM28 thermotolerance reversion warning and the Winter group’s data showing that BM28 is much less fit at 37 °C compared to the 37 °C-evolved strain and earlier heat evolved isolates, we believe that it is beneficial for cells to lose pOF39 when incubated at moderate temperatures, such as those used in P1 transduction experiments. Thus, we believe that like BM28, the strain that went on to become BM28 ΔlysU likely carried pOF39 at the end of its evolution but lost it in the P1 transduction process.
IS10R expansion theories and possible effects of IS10R insertion
Other unexpected findings were the extra 14–15 copies of IS10 scattered throughout the genomes. IS10s are non-replicative transposons, operating via a cut-and-paste mechanism. Thus, it is curious that a IS10 expansion event seems to have occurred in the BM28 strains, interrupting 10–11 genes and inserting into four intergenic regions (Fig. 5 and Table 1). However, non-replicative insertion sequences do increase in copy number in some descendants due to specific cut-and-paste events occurring during genome replication, and homologous recombination may play an additional role in some cases [48]. A study of the insertion sequence family IS4, which IS10s belong to, showed that substantial IS4 expansions events have occurred in some pathogens and extremophiles [49]. IS10 transposition is repressed at multiple levels and IS10 elements display multicopy suppression where the transposition activity decreases with increasing copies of Tn10/IS10 [50]. However, elevated temperatures [51], stationary phase culturing [52] and specific hemimethylation states of the DNA [53] can increase their transposition activity.
We believe that the insertion into the highly expressed metY-pnp operon (between infB and rbfA) is likely a contributor to the IS10R expansion (Fig. 5 and Table 1). metY-pnp is an eight gene operon which is transcribed into a number of polycistronic mRNAs. Three of the eight genes are clearly essential (nusA, infB, rpsO), and most of the products of these genes are present in the thousands per cell in MG1655 grown in complete media [54]. IS10R is inserted in the same orientation as the operon and would likely be present on a number of the operon’s polycistronic mRNAs. All together we think this IS10R insertion has the most potential for high transposase expression, and because of this we speculate that this IS10R may have been one of the first IS10R insertions in the strain, driving further insertions.
Noteworthy mutations in BM28 and BM28 ΔlysU
Through a combination of frame shifts, truncations, insertion sequence transpositions and large deletions, 223 genes were knocked out in BM28 and 220 genes were knocked out in BM28 ΔlysU (Supplementary Table S3 and Table 1). For 56 of these 223 BM28 genes, the melting temperatures of the proteins are known [36]. Fourteen of these 56 genes (25%) encode proteins with melting temperatures ≤ 5 °C above the Tmax of BM28 (≤ 53.5 °C) [36]. This proportion of the encoded proteins with melting temperatures ≤ 53.5 °C is comparable to the proportion of E. coli protein melting temperatures ≤ 53.5 °C determined by Mateus et al. (21.9%), thus, genes encoding low melting temperature proteins were not preferentially deleted in the BM28 strains [36]. Even so, it could be adaptive to delete nonessential genes encoding low melting temperature proteins if they are being expressed, to reduce protein unfolding or misfolding and subsequent protein aggregation in the cell at high incubation temperatures. An additional 14 BM28 and 15 BM28 ΔlysU genes encoding proteins with low melting temperatures had missense mutations (Additional File S2). When we ran these missense mutations together with published structures or Alphafold predicted structures [42] of the proteins through DDGun [40], the web-server predicted that six of the mutations increased protein stability, six of the mutations decreased stability and the remaining two BM28 and three BM28 ΔlysU mutations had no effect on stability (Additional File S2). When we performed the same analysis on missense mutations where the encoded protein melting temperatures were > 53.5 °C, we saw a similar distribution of predictions (18 stabilizing, 16 destabilizing and 9 neutral for BM28 and 17 stabilizing, 16 destabilizing and 8 neutral for BM28 ΔlysU). The strains also had changes in and around essential genes and heat shock genes (Tables 2 and 3), some of which (6 of 11 for BM28 and 5 of 10 for BM28 ΔlysU) were predicted to increase the stability of the proteins, which could clearly be helpful for growth at high temperature. Finally, a number of missense and silent mutations occurred in nonessential genes that are not heat induced (Additional File S2). It is important to note that even synonymous mutations can have substantial effects on proteins and organisms, including changes in substrate specificity [55], improved replication of a virus at high temperatures [56] and changes in organism fitness [57,58,59,60,61]. We will discuss select mutations that seem potentially important to growth at high temperature below.
Master regulators and transcription factors
Amongst the 223 or 220 inactivated genes are five confirmed transcription factors: cytR, gadW, fliZ (in BM28 only), nimR and slyA, which regulate 13, 15, 21, 2 and 37 genes, respectively [62]. These knockouts likely rewire transcription in BM28 and BM28 ΔlysU to some extent. Other transcription related proteins mutated in the strains include master regulators. Previous ALE studies, whether to heat or other stressors, identified changes in master regulators of transcription such as Rho and RpoB [91]. However, we must consider the tremendous distinction between survival to heat shock above the Tmax and what we are more interested in in heat ALE studies: growth at temperatures below or at the Tmax [91]. In BM28 and BM28 ΔlysU, especially near the rich liquid media Tmax of 48.5 °C, it could be that HslVU activity is essential for growth because the cells require an active protease for survival. HslVU’s presence could also be important due to its relationship with SulA, as we suspect that SulA and other SOS response proteins were expressed frequently in BM28 strains due to the IS10 insertions and large chromosomal deletions. If a shift away from σ32 and towards σ70 regulation leads to a more consistent expression of HslVU across many temperatures, perhaps this is adaptive for cells experiencing periodic DNA damage and higher incubation temperatures.
Conclusions
It is clear that BM28 strains followed interesting and unique evolutionary routes to their impressive Tmax’s. Chromosomal changes and the groESL plasmid pOF39 both seem to have contributed to their thermotolerance. Without pOF39, both BM28 and BM28 ΔlysU are still thermotolerant, but pOF39 was probably important for the strains during their evolution and it certainly contributes to their heat tolerance [9]. On the chromosomal side, we suspect that the master regulator and hslVU mutations are important to the thermotolerant phenotype. As well, we were able to clarify two points the Rudolph et al. paper is commonly cited for through WGS of the strains, without which we would also come to the original authors’ conclusions. Both centre around the groESL plasmid pOF39; BM28 overexpress GroESL due to the plasmid and not due to ALE to heat, and LysU does not seem to be important to thermotolerance in BM28 strains, rather, the pOF39 plasmid is. It will be interesting to examine other evolutionary routes towards increased thermotolerance in E. coli in future studies and to compare them with the few studies that have been published to date. In the future it is likely that researchers in this field will uncover more distinct routes to thermotolerance in E. coli and be able to classify the routes into general categories, to ultimately define how a bacterium can evolve to grow at higher temperatures. Bettering our understanding of enhancing thermotolerance in bacteria may have applications in enhancing thermotolerance in more complex organisms, especially those most affected by climate change and those important to humans or other organisms.
Experimental procedures
gDNA extraction and whole-genome sequencing
20–25 mL LB (1% w/v tryptone, 0.5% w/v yeast extract, 0.5% sodium chloride) cultures of BM28 and BM28 ΔlysU were grown up at 200–250 rpm overnight at 42–45 °C. Cells were harvested from the cultures, suspended in TE buffer (10 mM Tris, 1 mM EDTA), and lysed with proteases (Proteinase K or subtilisin) and 0.2–0.5% w/v sodium dodecyl sulphate at 37–50 °C. gDNA was extracted with 25:24:1 phenol:chloroform:isoamyl alcohol or guanidinium hydrochloride and ethanol, followed by cleanup on a silica column and elution in 10% v/v TE buffer (1 mM Tris, 0.1 mM EDTA). gDNA samples were sent to the Microbial Genome Sequencing Center in Pittsburgh for Illumina and Oxford Nanopore sequencing. For Illumina sequencing, 150 Mb sequencing packages were purchased for each sample (guaranteeing 32 X coverage for MG1655-derived BM28 and BM28 ΔlysU) and for Nanopore sequencing, Nanopore Only sequencing packages were purchased for each sample, which guaranteed a minimum of 300 Mb of sequencing data per sample (64 X coverage for MG1655-derived BM28).
Whole-genome sequencing data processing and analysis
For Illumina data, FastQ paired end read files were imported into Geneious using the default settings and Geneious automatically determined the read technology, so the only setting changed or inputted was that the insert size was set to 500 bp. Reads were trimmed using BBDuk with “trim adapters” selected with the default settings, “trim low quality” set to “both ends” with a minimum quality of 30, “trim adapters based on paired read overhangs” set to a minimum overlap of 24 and “discard short reads” set to a minimum length of 30. The reads were not normalized. The trimmed reads were mapped to the MG1655 reference genome or the JB41 draft genome depending on the purpose, using the default settings of map to reference with “do not trim” selected. Using the contig generated by map** the trimmed paired end reads to the JB41 draft genome reference, we found variations/SNPs using the default settings of “find variations/SNPs”. The variations/SNPs details were exported from Geneious Prime and analyzed in Microsoft Excel. For Nanopore data, FastQ files were imported into Geneious Prime and “Nanopore” was selected as the data type. Q30 trimmed Illumina paired end reads and raw nanopore reads were used for a SPAdes de novo assembly. The data source was set to “Multi Cell”, the method to “Assemble”, and the “Careful Mode” and “Do Not Trim” options were used. We compared the BM28 and BM28 ΔlysU genomes to the MG1655 reference genome with the progressive Mauve algorithm [23] set to default settings.
Identifying pOF39 in BM28, curing BM28 of pOF39 and transforming cells with pOF39
To initially detect pOF39 in BM28, extracted BM28 gDNA was transformed into chemically competent DH10B and the transformation was plated onto 1.5% w/v agar LB + Cb (100 µg/mL carbenicillin) plates. As well, primers specific to pOF39 sequences surrounding the groESL insert (forward primer: TTCAGCTGGATATTACGGCC, reverse primer: TGAGCGCATTGTTAGATTTCATAC) were used in a PCR with extracted BM28 gDNA as the template. BM28 and BM28 ΔlysU were plated from glycerol stocks onto LB and LB + Cb agar plates, incubated overnight at 37 °C, and growth on both plates were compared. A colony of BM28 from LB agar was subcultured in LB broth with 1% w/v sodium dodecyl sulphate every 24 h for three days, using a 100 X dilution. After the three days, cells were plated from the culture on LB agar and the plate was incubated at 37 °C overnight. The next day, single colonies were plated onto LB and LB + Cb agar and incubated at 37 °C overnight. Isolates which grew on LB but did not grow on LB + Cb were saved as presumptive pOF39-cured BM28. Plasmid DNA was prepared from BM28 that had been confirmed to carry pOF39, and the isolated plasmid DNA was used to transform MG1655 and BM28 ΔlysU.
High temperature growth experiments
MG1655, BM28 ΔlysU and BM28c (BM28 cured of pOF39) with and without pOF39, were plated at 37 °C on LB + Cb and LB plates, respectively. The next day, 2 mL LB aliquots in 16 mm glass test tubes were inoculated with several colonies of each sample, in quintuplicate, and incubated with shaking at 250 rpm in a water bath. Twenty-three hours later the final optical densities were measured and recorded. The experiment was performed at 46.3 °C and 47.8 °C. For high temperature plate growth experiments, the six isolates were plated for single colony isolation onto LB plates at 46.9–47.0 and 47.2–47.3 °C. Forty-eight hours later, plates were removed from the incubators and the isolates were scored for growth.
Motility assays
DH10B, MG1655, BM28 and BM28 ΔlysU (all lacking pOF39 except for BM28) were plated onto LB agar and incubated at 37 °C overnight. A single colony for each strain was picked with a sterile wooden stick and stabbed about two thirds of the way into the centre of 5 mL of soft LB agar (0.35% w/v agar) in a glass test tube and incubated overnight at 37 °C. The next day, the tubes were inspected for growth and scored as nonmotile if they grew at the edges of the stab and scored as motile if they grew throughout the soft agar. The experiment was performed in duplicate.
Availability of data and materials
All data are contained in the manuscript, the Additional Files, or submitted to GenBank. Raw short BM28 ΔlysU reads (SRR21641526), short BM28 reads (SRR21641525) and long BM28 reads (SRR21641524) were deposited to the SRA archive at Genbank, and final genome sequences were deposited in Genbank (JB41 CP102378.1, BM28 ΔlysU CP102379.1 and BM28 CP102380.1). All WGS files can be accessed from the Genbank BioProject PRJNA865726 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA865726). The pOF39 sequence (lacking the T —> C SNP) was also submitted to Genbank (OP156992.1, https://www.ncbi.nlm.nih.gov/nuccore/2310681246).
Abbreviations
- ALE:
-
Adaptive laboratory evolution
- gDNA:
-
Genomic DNA
- (p)ppGpp:
-
ppGpp and/or pppGpp
- RNAP:
-
RNA polymerase
- Tmax :
-
Maximum growth temperature
- Tmin :
-
Minimum growth temperature
- Topt :
-
Optimum growth temperature
- WGS:
-
Whole-genome sequencing
References
Hurst LD, Merchant AR. High guanine-cytosine content is not an adaptation to high temperature: a comparative analysis amongst prokaryotes. Proc R Soc B Biol Sci. 2001;268:493–7.
Jegousse C, Yang Y, Zhan J, Wang J, Zhou Y. Structural signatures of thermal adaptation of bacterial ribosomal RNA, transfer RNA, and messenger RNA. PLoS ONE. 2017;12:e0184722.
Koga Y. Thermal adaptation of the archaeal and bacterial lipid membranes. Archaea. 2012;2012:789652.
Siliakus MF, van der Oost J, Kengen SWM. Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles. 2017;21:651–70.
Goldstein RA. Amino-acid interactions in psychrophiles, mesophiles, thermophiles, and hyperthermophiles: Insights from the quasi-chemical approximation. Protein Sci. 2007;16:1887–95.
McDonald JH. Temperature adaptation at homologous sites in proteins from nine thermophile-mesophile species pairs. Genome Biol Evol. 2010;2:267–76.
Sadeghi M, Naderi-Manesh H, Zarrabi M, Ranjbar B. Effective factors in thermostability of thermophilic proteins. Biophys Chem. 2006;119:256–70.
Szilágyi A, Závodszky P. Structural differences between mesophilic, moderately thermophilic and extremely thermophilic protein subunits: results of a comprehensive survey. Structure. 2000;8:493–504.
Rudolph B, Gebendorfer KM, Buchner J, Winter J. Evolution of Escherichia coli for growth at high temperatures. J Biol Chem. 2010;285:19029–34.
Blaby IK, Lyons BJ, Wroclawska-Hughes E, Phillips GCF, Pyle TP, Chamberlin SG, et al. Experimental evolution of a facultative thermophile from a mesophilic ancestor. Appl Environ Microbiol. 2012;78:144–55.
Luan G, Bao G, Lin Z, Li Y, Chen Z, Li Y, et al. Comparative genome analysis of a thermotolerant Escherichia coli obtained by Genome Replication Engineering Assisted Continuous Evolution (GREACE) and its parent strain provides new understanding of microbial heat tolerance. N Biotechnol. 2015;32:732–8.
Kosaka T, Nakajima Y, Ishii A, Yamashita M, Yoshida S, Murata M, et al. Capacity for survival in global warming: Adaptation of mesophiles to the temperature upper limit. PLoS ONE. 2019;14:e0215614.
Kishimoto T, Iijima L, Tatsumi M, Ono N, Oyake A, Hashimoto T, et al. Transition from positive to neutral in mutation fixation along with continuing rising fitness in thermal adaptive evolution. PLoS Genet. 2010;6:1–10.
Zhang T, Shi X-C, **a Y, Mai L, Tremblay P-L. Escherichia coli adaptation and response to exposure to heavy atmospheric pollution. Sci Rep. 2019;9:10879.
Tenaillon O, Rodríguez-Verdugo A, Gaut RL, McDonald P, Bennett AF, Long AD, et al. The molecular diversity of adaptive convergence. Science. 2012;335:457–61.
González-González A, Hug SM, Rodríguez-Verdugo A, Patel JS, Gaut BS. Adaptive mutations in RNA polymerase and the transcriptional terminator rho have similar effects on Escherichia coli gene expression. Mol Biol Evol. 2017;34:2839–55.
Rodŕiguez-Verdugo A, Tenaillon O, Gaut BS. First-Step mutations during adaptation restore the expression of hundreds of genes. Mol Biol Evol. 2016;33:25–39.
Sandberg TE, Pedersen M, Lacroix RA, Ebrahim A, Bonde M, Herrgard MJ, et al. Evolution of Escherichia coli to 42 C and subsequent genetic engineering reveals adaptive mechanisms and novel mutations. Mol Biol Evol. 2014;31:2647–62.
Gur E, Biran D, Gazit E, Ron EZ. In vivo aggregation of a single enzyme limits growth of Escherichia coli at elevated temperatures. Mol Microbiol. 2002;46:1391–7.
Mordukhova EA, Lee HS, Pan JG. Improved thermostability and acetic acid tolerance of Escherichia coli via directed evolution of homoserine o-succinyltransferase. Appl Environ Microbiol. 2008;74:7660–8.
Mordukhova EA, Kim D, Pan JG. Stabilized homoserine o-succinyltransferases (MetA) or L-methionine partially recovers the growth defect in Escherichia coli lacking ATP-dependent proteases or the DnaK chaperone. BMC Microbiol. 2013;13:179.
Bardwell JC, Craig EA. Ancient heat shock gene is dispensable. J Bacteriol. 1988;170:2977–83.
Darling AE, Mau B, Perna NT. ProgressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 2010;5:e11147.
Wang X, Kim Y, Ma Q, Hong SH, Pokusaeva K, Sturino JM, et al. Cryptic prophages help bacteria cope with adverse environments. Nat Commun. 2010;1:147.
Jeong H, Barbe V, Lee CH, Vallenet D, Yu DS, Choi SH, et al. Genome Sequences of Escherichia coli B strains REL606 and BL21(DE3). J Mol Biol. 2009;394:644–52.
Stothard P, Wishart DS. Circular genome visualization and exploration using CGView. Bioinformatics. 2005;21:537–9.
Drake JW. A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci U S A. 1991;88:7160–4.
Fayet O, Louarn J-M, Georgopoulos C. Suppression of the Escherichia coli dnaA46 mutation by amplification of the groES and groEL genes. MGG Mol Gen Genet. 1986;202:435–45.
La Fleur T, Hossain A, Salis HM. Automated model-predictive design of synthetic promoters to control transcriptional profiles in bacteria. bioRxiv. 2021. https://doi.org/10.1101/2021.09.01.458561.
Fahnert B, Lilie H, Neubauer P. Inclusion bodies: formation and utilisation. In: Advances in biochemical engineering/biotechnology. 2004. p. 93–142.
Cornet I, Van Derlinden E, Cappuyns AM, Van Impe JF. Heat stress adaptation of Escherichia coli under dynamic conditions: effect of inoculum size. Lett Appl Microbiol. 2010;51:450–5.
Van Derlinden E, Bernaerts K, Van Impe JF. Unravelling Escherichia coli dynamics close to the maximum growth temperature through heterogeneous modelling. Lett Appl Microbiol. 2009;49:659–65.
Van Derlinden E, Lule I, Bernaerts K, Van Impe JF. Quantifying the heterogeneous heat response of Escherichia coli under dynamic temperatures. J Appl Microbiol. 2010;108:1123–35.
Foster TJ, Davis MA, Roberts DE, Takeshita K, Kleckner N. Genetic organization of transposon Tn10. Cell. 1981;23:201–13.
Bender J, Klecknert N. Tn10 insertion specificity is strongly dependent upon sequences immediately adjacent to the target-site consensus sequence. Proc Natl Acad Sci. 1992;89:7996–8000.
Mateus A, Bobonis J, Kurzawa N, Stein F, Helm D, Hevler J, et al. Thermal proteome profiling in bacteria: probing protein state in vivo. Mol Syst Biol. 2018;14:e8242.
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006.0008.
Yamamoto N, Nakahigashi K, Nakamichi T, Yoshino M, Takai Y, Touda Y, et al. Update on the Keio collection of Escherichia coli single-gene deletion mutants. Mol Syst Biol. 2009;5:335.
Goodall ECAA, Robinson A, Johnston IG, Jabbari S, Turner KA, Cunningham AF, et al. The essential genome of Escherichia coli K-12. MBio. 2018;9:1–18.
Montanucci L, Capriotti E, Birolo G, Benevenuta S, Pancotti C, Lal D, et al. DDGun: an untrained predictor of protein stability changes upon amino acid variants. Nucleic Acids Res. 2022. https://doi.org/10.1093/nar/gkac325.
Nonaka G, Blankschien M, Herman C, Gross CA, Rhodius VA. Regulon and promoter analysis of the E. coli heat-shock factor, sigma32, reveals a multifaceted cellular response to heat stress. Genes Dev. 2006;20:1776–89.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9.
Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2018;47:419–26.
Carbon S, Douglass E, Good BM, Unni DR, Harris NL, Mungall CJ, et al. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49:D325–34.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25:25–9.
Hecht A, Glasgow J, Jaschke PR, Bawazer LA, Munson MS, Cochran JR, et al. Measurements of translation initiation from all 64 codons in E. coli. Nucleic Acids Res. 2017;45:3615–26.
Chayot R, Montagne B, Mazel D, Ricchetti M. An end-joining repair mechanism in Escherichia coli. Proc Natl Acad Sci U S A. 2010;107:2141–6.
Rowley PA, Kachroo AH, Jayaram M. Selfish DNA. Brenner’s Encycl Genet Second Ed. 2013;4:382–9.
De Palmenaer D, Siguier P, Mahillon J. IS4 family goes genomic. BMC Evol Biol. 2008;8:18.
Simons RW, Kleckner N. Translational control of IS10 transposition. Cell. 1983;34:683–91.
Pepe CM, Masleša-Galić S, Simons RW. Decay of the IS 10 antisense RNA by 3′ exoribonucleases: evidence that RNase II stabilizes RNA-OUT against PNPase attack. Mol Microbiol. 1994;13:1133–42.
Munshaw BJ, Haniford D. The regulation of prokaryotic transposable elements by the RNA binding protein Hfq. 2012.
Roberts D, Hoopes BC, McClure WR, Kleckner N. IS10 transposition is regulated by DNA adenine methylation. Cell. 1985;43:117–30.
Li GW, Burkhardt D, Gross C, Weissman JS. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell. 2014;157:624–35.
Kimchi-Sarfaty C, Oh JM, Kim I-W, Sauna ZE, Calcagno AM, Ambudkar SV, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;318:1382–3.
Kashiwagi A, Sugawara R, Sano Tsushima F, Kumagai T, Yomo T. Contribution of silent mutations to thermal adaptation of RNA bacteriophage Qβ. J Virol. 2014;88:11459–68.
Hauber DJ, Grogan DW, DeBry RW. Mutations to less-preferred synonymous codons in a highly expressed gene of Escherichia coli: Fitness and epistatic interactions. PLoS ONE. 2016;11:e0146375.
Kristofich JC, Morgenthaler AB, Kinney WR, Ebmeier CC, Snyder DJ, Old WM, et al. Synonymous mutations make dramatic contributions to fitness when growth is limited by a weak-link enzyme. PLoS Genet. 2018;14:e1007615.
Agashe D, Sane M, Phalnikar K, Diwan GD, Habibullah A, Martinez-Gomez NC, et al. Large-effect beneficial synonymous mutations mediate rapid and parallel adaptation in a bacterium. Mol Biol Evol. 2016;33:1542–53.
Knöppel A, Näsvall J, Andersson DI. Compensating the fitness costs of synonymous mutations. Mol Biol Evol. 2016;33:1461–77.
Bailey SF, Hinz A, Kassen R. Adaptive synonymous mutations in an experimentally evolved Pseudomonas fluorescens population. Nat Commun. 2014;5:4076.
Santos-Zavaleta A, Salgado H, Gama-Castro S, Sánchez-Pérez M, Omez-Romero LG, Ledezma-Tejeida D, et al. RegulonDB v 10.5: tackling challenges to unify classic and high throughput knowledge of gene regulation in E. coli K-12. Nucleic Acids Res. 2019;47:D212–20.
Hao Z, Epshtein V, Kim KH, Mironov A, Walz T, Correspondence EN, et al. Pre-termination transcription complex: structure and function. Mol Cell. 2021;81:281-292.e8.
Molodtsov V, Sineva E, Zhang L, Huang X, Cashel M, Ades SE, et al. Allosteric effector ppGpp potentiates the inhibition of transcript initiation by DksA. Mol Cell. 2018;69:828-839.e5.
Chen J, Gopalkrishnan S, Chiu C, Chen AY, Campbell EA, Gourse RL, et al. E. coli TraR allosterically regulates transcription initiation by altering RNA polymerase conformation. Elife. 2019;8:1–29.
Blankschien MD, Lee JH, Grace ED, Lennon CW, Halliday JA, Ross W, et al. Super DksAs: substitutions in DksA enhancing its effects on transcription initiation. EMBO J. 2009;28:1720–31.
Gray MJ, Wholey WY, Wagner NO, Cremers CM, Mueller-Schickert A, Hock NT, et al. Polyphosphate is a primordial chaperone. Mol Cell. 2014;53:689–99.
Song H, Dharmasena MN, Wang C, Shaw GX, Cherry S, Tropea JE, et al. Structure and activity of PPX/GppA homologs from Escherichia coli and Helicobacter pylori. FEBS J. 2020;287:1865–85.
Kappler U, Roghanian M, Zhang C-C, Dasgupta S, Rakshit D, Das B, et al. Functional Insights Into the Role of gppA in (p)ppGpp metabolism of vibrio cholerae. Front Microbiol. 2020;11:564644.
Pedersen H, Sogaard-Andersen L, Holst B, Valentin-Hansen P. Heterologous cooperativity in Escherichia coli: the CytR repressor both contacts DNA and the cAMP receptor protein when binding to the deoP2 promoter. J Biol Chem. 1991;266:17804–8.
Lauritsen I, Frendorf PO, Capucci S, Heyde SAH, Blomquist SD, Wendel S, et al. Temporal evolution of master regulator Crp identifies pyrimidines as catabolite modulator factors. https://doi.org/10.1038/s41467-021-26098-x.
Kallipolitis BH, Valentin-Hansen P. Transcription of rpoH, encoding the Escherichia coli heat-shock regulator σ32, is negatively controlled by the cAMP-CRP/CytR nucleoprotein complex. Mol Microbiol. 1998;29:1091–9.
Borujeni AE, Cetnar D, Farasat I, Smith A, Lundgren N, Salis HM. Precise quantification of translation inhibition by mRNA structures that overlap with the ribosomal footprint in N-terminal coding sequences. Nucleic Acids Res. 2017;45:5437–48.
Levin-Karp A, Barenholz U, Bareia T, Dayagi M, Zelcbuch L, Antonovsky N, et al. Quantifying translational coupling in E. coli synthetic operons using RBS modulation and fluorescent reporters. ACS Synth Biol. 2013;2:327–36.
Straus DB, Walter WA, Gross CA. The heat shock response of E. coli is regulated by changes in the concentration of σ32. Nature. 1987;329:348–51.
Batarseh TN, Hug SM, Batarseh SN, Gaut BS. Genetic mutations that drive evolutionary rescue to lethal temperature in Escherichia coli. Genome Biol Evol. 2020;12:2029–44.
Katayama T, Kubota T, Takata M, Akimitsu N, Sekimizu K. Disruption of the hslU gene, which encodes an ATPase subunit of the eukaryotic 26S proteasome homolog in Escherichia coli, suppresses the temperature-sensitive dnaA46 mutation. Biochem Biophys Res Commun. 1996;229:219–24.
Murata M, Fujimoto H, Nishimura K, Charoensuk K, Nagamitsu H, Raina S, et al. Molecular strategy for survival at a critical high temperature in Escherichia coli. PLoS ONE. 2011;6:20063.
Murata M, Ishii A, Fujimoto H, Nishimura K, Kosaka T, Mori H, et al. Update of thermotolerant genes essential for survival at a critical high temperature in Escherichia coli. PLoS ONE. 2018;13:1–10.
Deatherage DE, Kepner JL, Bennett AF, Lenski RE, Barrick JE. Specificity of genome evolution in experimental populations of Escherichia coli evolved at different temperatures. Proc Natl Acad Sci U S A. 2017;114:E1904–12.
Campos M, Govers SK, Irnov I, Dobihal GS, Cornet F, Jacobs-Wagner C. Genomewide phenotypic analysis of growth, cell morphogenesis, and cell cycle events in Escherichia coli. Mol Syst Biol. 2018;14:e7573.
Kanemori M, Yanagi H, Yura T. Marked instability of the σ32 heat shock transcription factor at high temperature. Implications for heat shock regulation. J Biol Chem. 1999;274:22002–7.
Lee YY, Chang CF, Kuo CL, Chen MC, Hung YuC, Lin PI, et al. Subunit oligomerization and substrate recognition of the Escherichia Coli ClpYQ (HslUV) protease implicated by in vivo protein-protein interactions in the yeast two-hybrid system. J Bacteriol. 2003;185:2393–401.
Tsai CH, Ho YH, Sung TC, Wu WF, Chen CS. Escherichia coli proteome microarrays identified the substrates of ClpYQ protease. Mol Cell Proteomics. 2017;16:113–20.
Kanemori M, Nishihara K, Yanagi H, Yura T. Synergistic roles of HslVU and other ATP-dependent proteases in controlling in vivo turnover of σ32 and abnormal proteins in Escherichia coli. J Bacteriol. 1997;179:7219–25.
Yura T. Regulation of the heat shock response in Escherichia coli: history and perspectives. Genes Genet Syst. 2019;94:103–8.
Sousa MC, Trame CB, Tsuruta H, Wilbanks SM, Reddy VS, McKay DB. Crystal and solution structures of an HslUV protease-chaperone complex. Cell. 2000;103:633–43.
Seong IS, Oh JY, Lee JW, Tanaka K, Chung CH. The HslU ATPase acts as a molecular chaperone in prevention of aggregation of SulA, an inhibitor of cell division in Escherichia coli. FEBS Lett. 2000;477:224–9.
Kanemori M, Yanagi H, Yura T. The ATP-dependent HslVU/ClpQY protease participates in turnover of cell division inhibitor SulA in Escherichia coli. J Bacteriol. 1999;181:3674–80.
Burton RE, Baker TA, Sauer RT. Nucleotide-dependent substrate recognition by the AAA+ HslUV protease. Nat Struct Mol Biol. 2005;12:245–51.
Govers SK, Mortier J, Adam A, Aertsen A. Protein aggregates encode epigenetic memory of stressful encounters in individual Escherichia coli cells. 2018;16:8.
Acknowledgements
We thank Dr. Dragana Despotović, Dr. Dan Tawfik, Dr. Johannes Buchner and other researchers that helped us track down the BM28 strains.
Funding
This work was supported by grants from Natural Sciences and Engineering Research Council of Canada (RGPIN-2018–03747).
Author information
Authors and Affiliations
Contributions
BM performed the experiments, processed and analyzed the WGS data and wrote the manuscript. BM and FN edited the manuscript. FN supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table S1. BM28 and BM28 ΔlysU NextSeq 2000 Illumina WGS data statistics. Supplementary Table S2. BM28 Oxford Nanopore WGS data statistics. Supplementary Table S3. Large deletions in BM28 and BM28 ΔlysU. Supplementary Table S4. BM28 and BM28 ΔlysU mutation details. Supplementary Table S5. High temperature growth scores of BM28-related cells with and without pOF39. Supplementary Table S6. Smaller BM28 and BM28 ΔlysU indels. Supplementary Table S7. PANTHER Overrepresentation Test of BM28 mutations using the Annotation Data Set GO cellular component complete. Supplementary Table S8. PANTHER Overrepresentation Test of BM28 ΔlysU mutations using the Annotation Data Set GO cellular component complete. Supplementary Table S9. Motility of DH10B, MG1655, BM28 and BM28 ΔlysU. Supplementary Figure S1. The 12 bp of homology between dinB and mhpE. Supplementary Figure S2. The O-antigen deletion in BM28 and BM28 ΔlysU. Supplementary Figure S3. BM28 and BM28 ΔlysU SNP mutation spectra. Supplementary Figure S4. Differences between BM28 and BM28 ΔlysU. Supplementary Figure S5. Growth of BM28 and BM28 ΔlysU on LB agar (left) and LB + Cb agar (right). Supplementary Figure S6. DH10B BM28 gDNA transformation plate. Supplementary Figure S7. PCR of BM28 gDNA using pOF39-specific primers. Supplementary Figure S8. Alignment of wildtype IS10R, wildtype IS10L and the IS10L/R hybrid from the BM28 Tn10. Supplementary Figure S9. Alignment of wildtype IS10R, wildtype IS10L and the IS10L/R hybrid from the BM28 ΔlysU Tn10. Supplementary Figure S10. Sequence logo of the IS10R target sequence, made using WebLogo (https://weblogo.berkeley.edu/logo.cgi). Supplementary Figure S11. Rho T96 is located on the surface of the protein and does not contact RNA polymerase nor other Rho monomers. Supplementary Figure S12. RpoC A595 is located on the surface of the protein and does not contact other RNA polymerase components, nor Rho. Supplementary Figure S13. RpoC T1135 is in close proximity to DksA, namely, DksA residue D90 (the closest distance, represented by a pink dotted line, is 7.1 Å). Supplementary Figure S14. RpoC T1135, DksA and a ppGpp molecule are fairly close to the active site magnesium ion of the RNA polymerase.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
McGuire, B.E., Nano, F.E. Whole-genome sequencing analysis of two heat-evolved Escherichia coli strains. BMC Genomics 24, 154 (2023). https://doi.org/10.1186/s12864-023-09266-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09266-9