Abstract
Background
A body distribution with high intramuscular fat and low abdominal fat is the ideal goal for broiler breeding. Preadipocytes with different origins have differences in terms of metabolism and gene expression. The transcriptome analysis performed in this study of intramuscular preadipocytes (DIMFPs) and adipose tissue-derived preadipocytes (DAFPs) aimed to explore the characteristics of lipid deposition in different chicken preadipocytes by dedifferentiation in vitro.
Results
Compared with DAFPs, the total lipid content in DIMFPs was reduced (P < 0.05). Moreover, 72 DEGs related to lipid metabolism were screened, which were involved in adipocyte differentiation, fatty acid transport and fatty acid synthesis, lipid stabilization, and lipolysis. Among the 72 DEGs, 19 DEGs were enriched in the PPAR signaling pathway, indicating its main contribution to the regulation of the difference in lipid deposition between DAFPs and DIMFPs. Among these 19 genes, the representative APOA1, ADIPOQ, FABP3, FABP4, FABP7, HMGCS2, LPL and RXRG genes were downregulated, but the ACSL1, FABP5, PCK2, PDPK1, PPARG, SCD, SCD5, and SLC27A6 genes were upregulated (P < 0.05 or P < 0.01) in the DIMFPs. In addition, the well-known pathways affecting lipid metabolism (MAPK, TGF-beta and calcium) and the pathways related to cell communication were enriched, which may also contribute to the regulation of lipid deposition. Finally, the regulatory network for the difference in lipid deposition between chicken DAFPs and DIMFPs was proposed based on the above information.
Conclusions
Our data suggested a difference in lipid deposition between DIMFPs and DAFPs of chickens in vitro and proposed a molecular regulatory network for the difference in lipid deposition between chicken DAFPs and DIMFPs. The lipid content was significantly increased in DAFPs by the direct mediation of PPAR signaling pathways. These findings provide new insights into the regulation of tissue-specific fat deposition and the optimization of body fat distribution in broilers.
Similar content being viewed by others
Background
Fat has unique distribution characteristics and different economic values in various tissues of animals. In broilers, high-intensity artificial breeding has effectively increased the meat yield but has also increased the abdominal fat content and reduced intramuscular fat deposition [1]. Excessive abdominal fat deposition has negative impacts on the feed efficiency and carcass yield [2, 3]. Decreased abdominal fat deposition is beneficial to reduce waste and improve consumer acceptance. In contrast, intramuscular fat is economically desirable in broiler production. Appropriately increased IMF content can improve the meat quality, including color, tenderness, flavor, and juiciness [4,5,6,7]. Lowering abdominal fat and increasing intramuscular fat can effectively increase the economic value of broilers.
Previous studies have shown that adipocytes with different origins exhibit differential differentiation capabilities [8]. Compared with subcutaneous preadipocytes, the cell size and lipid droplets in intramuscular adipocytes are smaller [9, 10], and the gene expression and enzyme activation related to lipid metabolism are lower in intramuscular adipocytes [11, 12]. Similarly, abdominal fat-derived preadipocytes exhibited a higher adipogenic differentiation ability than intramuscular fat-derived preadipocytes in chickens [13, 14]. However, it is still unknown whether the difference in the lipogenesis ability of preadipocytes from different tissues will disappear after cultivation in vitro.
In this study, we explored the lipogenesis characteristics of chicken preadipocytes of different origins after cultivation in vitro, including dedifferentiated intramuscular preadipocytes (DIMFPs) and dedifferentiated abdominal preadipocytes (DAFPs). These results will help to understand tissue-specific lipid deposition and optimize body fat distribution in broilers.
Results
The difference in lipid deposition in the two types of preadipocytes
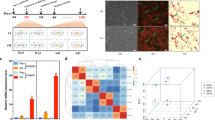
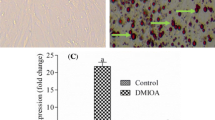
Collect the DIMFP group and DAFP group cells were collected to detect the total lipid content by an Oil Red O staining assay. As shown in Fig. 1a, the total lipid content in DAFP cells was significantly (P < 0.05) higher than that in DIMFP cells. The main ingredients of lipids, triglycerides (TGs), phospholipids (PLIPs), and total cholesterol (TCHO) were also detected. Similarly, the TG content in DAFP cells was significantly (P < 0.05) higher than that in DIMFP cells. However, the contents of PLIP and TCHO showed no difference in the two types of preadipocytes (Fig. 1b).
Difference in lipid metabolism between DIMFPs and DAFPs of chickens. a and b The contents of total lipids and the main ingredients of lipids (TG, PLIP and TCHO). The total lipid and TG contents were increased in the DAFPs compared with the DIMFPs after two days at 100% confluence. Data are presented as the means ± SEM (n = 3; * P < 0.05)
Identification of DEGs
Total RNA of each of the three cell repetitions of the DIMFP and DAFP groups was extracted for RNA sequencing. A total of 21,469 expressed genes were found in DIMFPs and DAFPs (Additional file 1: Table S1). Using gene expression profiling and comparing the DAFP group with the DIMFP group (DIMFP vs DAFP), a total of 3629 known DEGs (|log2 FC| ≥1, with P < 0.05) were screened (Fig. 2a), of which 2579 DEGs were downregulated and 907 DEGs were upregulated (Additional file 2: Table S2). Next, cluster analysis was performed on these 21,469 genes, and two results showed the same situation: three cell samples of the same groups were clustered together (Fig. 2b).
Volcano plot and cluster analysis of differentially expressed genes (DEGs). a Volcano plot. Red dots (UP) represent significantly upregulated genes (log2FC ≥ 1.0, FDR < 0.05); blue dots (DOWN) represent significantly downregulated genes (log2 FC ≤ − 1.0, FDR < 0.05); and black dots (NO) represent DEGs below the level of significance; (b) based on 3486 known DEGs in DIMFPs and DAFPs of chickens, cluster analysis was performed. The results show that the gene expression profiling data in the same group were closely related
Analysis of the enriched GO terms and pathways in the two types of preadipocytes
Based on 3629 known DEGs, Gene Ontology (GO) analysis was performed, and 56 GO terms were enriched (P < 0.05), mainly including the following processes: cell adhesion, tight adhesion, cell differentiation, extracellular matrix, DNA binding, calcium ion binding, etc. (Additional file 3: Table S3). The top 10 terms of each of the biological process (BP), cellular component (CC) and molecular function (MF) terms are shown in Fig. 3.
Meanwhile, 47 pathways were found to be significantly enriched (corrected P-value < 0.05) (Additional file 4: Table S4), including some well-known pathways affecting lipid metabolism (PPAR, MAPK, TGF-beta, Wnt, and calcium signaling pathways) and other pathways related to cell communication (focal adhesion, cytokine-cytokine receptor interaction, ECM-receptor interaction, tight junction, regulation of the actin cytoskeleton, cell adhesion molecules, and adherens junction pathways). The top 15 enriched pathways are shown in Fig. 4.
List of enriched pathways with the top 15 based on the 3486 DEGs. The KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis showed that well-known pathways (MAPK, TGF-beta, Wnt, calcium, and PPAR signaling pathways) of lipid metabolism were enriched, and the enriched pathways with the top 15 were screened (adjusted P < 0.05)
DEGs related to lipid metabolism in the two types of preadipocytes
GO enrichment analysis indicated 72 DEGs related to lipid metabolism, and some representative DEGs were screened (Additional file 5: Table S5). The DEGs related to lipid metabolism were mainly involved in adipocyte differentiation (such as CEBPA, PPARG, RBP7, and RXRG), fatty acid transport and fatty acid synthesis (such as ELOVL1, ELOVL6, FABP3, FABP4, FADS6, FADS1 L1, SCD, and SCD5), lipid stabilization (such as CIDEC, PLIN3, PLIN4, and MOGAT1), and lipolysis (such as DGKD, DGKH, DGKQ, and LPL). The 20 representative DEGs related to lipid metabolism were randomly selected to validate the gene expression profiling results by qRT-PCR, and the correlation of gene expression profiling and qRT-PCR was analyzed by Spearman rank correlation to confirm the accuracy of the data. The results showed that the fold change in gene expression between the two methods was significantly correlated (Fig. 5a) (r = 0.9666, P < 0.01).
Validation of DEGs related to lipid metabolism between DIMFPs and DAFPs of chickens. a Correlation analysis of gene expression profiling and real-time quantitative polymerase chain reaction (qRT-PCR) results by Spearman rank correlation in DIMFPs and DAFPs. A high correlation coefficient (r = 0.9666, P < 0.05) was detected, which indicates that the gene expression profiling data are reliable. n = 20; (b) and (c) qRT-PCR verification of DEGs detected by gene expression profiling. The expression levels of DEGs related to lipid metabolism determined by qRT-PCR in the DIMFPs and DAFPs. Each of these DEGs was upregulated or downregulated significantly (P < 0.05) in DIMFPs and DAFPs. Data are presented as the means ± SEM (n = 3; * P < 0.05, ** P < 0.01)
Among these 20 verified genes, the expression levels of the CEBPA, DGKH, DGKQ, DGKD, FADS1L1, SCD, SCD5, and PPARG genes were significantly (P < 0.05 or P < 0.01) downregulated in DAFPs compared to DIMFPs (Fig. 5b). However, the expression levels of the CIDEC, ELOVL1, ELOVL6, FABP3, FABP4, FADS6, LPL, MOGAT1, PLIN3, PLIN4, RBP7, and RXRG genes were significantly (all P < 0.01) upregulated in DAFPs compared to DIMFPs (Fig. 5c).
Pathways involved in lipid metabolism
It was found that 19 genes related to lipid metabolism enriched in the PPAR signaling pathway (Additional file 6: Fig. S1). Among these 19 genes, the data from RNA-seq showed that APOA1, ADIPOQ, FABP3, FABP4, FABP7, HMGCS2, LPL and RXRG genes were down-regulated, but ACSL1, FABP5, PCK2, PDPK1, PPARG, SCD, SCD5, SLC27A6 genes were up-regulated (P < 0.05 or P < 0.01) in the DIMFPs. (Additional file 2: Table S2).
Also, there are a large number of DEGs that were enriched in MAPK- (80 genes), Calcium- (50 genes), and TGF beta (30 genes) signaling pathway, which involved in mediating the biology function of lipid metabolism (Additional file 7: Fig. S2, Additional file 8: Fig. S3, and Additional file 9: Fig. S4). Besides, 245 DEGs also were enriched the pathways related to cell communications (Focal adhesion, Cytokine-cytokine receptor interaction, ECM-receptor interaction, Tight junction, Regulation of actin cytoskeleton, cell adhesion molecules, Adherens junction). However, it was found that the enriched Wnt signaling pathway, as a well-known pathway affecting lipid metabolism, did not medicate the regulation of lipid metabolism. Based on the above information, we proposed the regulatory network for the difference of lipid deposition between chicken DAFPs and DIMFPs (Fig. 6).
Discussion
Fat has unique distribution characteristics and different economic values in various tissues of animals. In broilers, intramuscular fat is economically desirable in production. Appropriately increased IMF content can improve meat quality, including tenderness, flavor, and juiciness [4,5,6]. However, excessive abdominal fat deposition has negative impacts on the feed efficiency and carcass yield [2, 3], and decreased abdominal fat deposition is beneficial to reduce waste production and improve consumer acceptance. Lowering abdominal fat and increasing intramuscular fat can effectively increase the economic value of broilers. Therefore, changing the constitution distribution is an important scientific problem for broilers.
Unlike the marbling distribution of IMF in domestic animals, the IMF of chickens cannot be obtained directly from anatomy. Moreover, chicken muscle tissue has a variety of cell compositions [15], and IMF preadipocytes cannot be separated by physical methods due to their similar density to muscle cells. Therefore, high-purity preadipocytes of IMF can only be obtained by the dedifferentiation of mature adipocytes in vitro as described previously [16]. In this study, abdominal fat preadipocytes and intramural preadipocytes were obtained from mature adipocytes of the same chicken to compare their lipogenesis ability under consistent experimental conditions in vitro, establishing a theoretical foundation for the body fat distribution of chickens and providing ideas and development directions for chicken production.
Adipocytes in different tissues are regulated by the adjacent microenvironment to perform the corresponding physiological function [40]. The edgeR R software package was used to analyze the differentially expressed genes (DEGs) between the DIMFP group and the DAFP group. The DEG screening conditions were | log2 FC | ≥1.0 and FDR < 0.05. Cluster analysis of DEGs was performed by the pheatmap software package of R software.
Gene ontology and Kyoto encyclopedia of genes and genomes analysis
The ClueGO plug-in and CluePedia plug-in in Cytoscape (https://cytoscape.org/) software were used to perform Gene Ontology (GO) enrichment analysis and functional classification of the DEGs. The significance level of GO term enrichment was set at P < 0.05. At the same time, the Kyoto Encyclopedia of Genes and Genomes (KEGG) [41] database was used for pathway enrichment analysis of DEGs. P < 0.05 was considered to be indicative of statistical significance. According to the results of GO enrichment analysis and KEGG pathway function enrichment, DEGs related to abdominal fat tissue metabolism were screened.
Real-time quantitative polymerase chain reaction
A Tiangen® FastQuant RT kit (Tiangen, Bei**g, China) was used for the reverse transcription of the RNA samples of the two groups of cells according to the steps provided by the manufacturer’s instructions, and Primer 5.0 software was used to design specific primers for each gene according to the GenBank sequence (Additional file 10: Table S6). The ABI 7500 real-time PCR detection system (Applied Biosystems, CA, USA) was used for relative quantitative analysis of gene expression in different samples. The total reaction system was 20 μL and contained 10 μL of SYBR Green mix (TaKaRa, Shanghai, China), 0.5 μL of forward primers, and 0.5 μL of reverse primers at a concentration of 10 nmol, 1 μL of cDNA, and 8 μL of ddH2O. The reaction program was denaturation at 95 °C for 30 s, followed by 40 cycles of amplification at 95 °C for 5 s and 60 °C for 32 s. The 2-△△Ct method [42] was used to calculate the fold change of gene expression with the expression of actin beta (β-actin), which was a reference gene.
Statistical analysis
Three comparison replicates (DIMFPs vs DAFPs) of the cell experiment were set according to the one-to-one correspondence of cell samples from the abdominal fat tissue and pectoralis major of the same chicken. All experiments were repeated three times, and the data obtained are expressed as the mean ± SEM. Statistically significant differences between the two culture conditions were tested by independent-samples t-tests using SAS 9.2 software (SAS Institute Inc., NC, USA). P < 0.05 (*) or P < 0.01 (**) was considered to be significant. All figures were constructed using GraphPad Prism version 5.02 (GraphPad Software Inc., CA, USA).
Availability of data and materials
All of raw data generated during the study are uploaded to the NCBI-SRA database (https://www.ncbi.nlm.nih.gov/sra/) under the accession number SRR13665175, SRR13665173, SRR13665172, SRR13665171, SRR13665170, SRR13665174.
Abbreviations
- ACSL1:
-
Acyl-CoA synthetase long-chain family member 1
- APOA1:
-
Apolipoprotein A1
- ADIPOQ:
-
Adiponectin, C1Q and collagen domain containing
- BP:
-
Biological process
- CC:
-
Cellular component
- CEBP:
-
CCAAT enhancer binding protein
- CIDEC:
-
Cell death inducing DFFA like effector c
- DEG:
-
Differentially expressed genes
- DIMFPs:
-
Dedifferentiated intramuscular preadipocytes
- DAFPs:
-
Dedifferentiated abdominal preadipocytes
- DGK:
-
Diacylglycerol kinase
- ELOVL:
-
Elongase of very long-chain fatty acids-like
- FABP:
-
Fatty acid binding protein
- FADS:
-
Fatty acid desaturase
- HMGCS2:
-
3-Hydroxy-3-methylglutaryl-coenzyme a synthase 2
- IMF:
-
Intramuscular fat
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- LPL:
-
Lipoprotein lipase
- MAPK:
-
Mitogen- activated protein kinase
- MF:
-
Molecular function
- MOGAT:
-
Monoacylglycerol O-acyltransferase.
- PBS:
-
Phosphate-buffered saline
- PCK2:
-
Phosphoenolpyruvate Carboxykinase 2
- PDPK1:
-
3-Phosphoinositide dependent protein kinase-1
- PLIN:
-
Perilipin
- PPAR:
-
Peroxisome proliferators-activated receptors
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- RBP:
-
Retinol binding protein
- RXR:
-
Retinoid X receptor
- SCD:
-
Stearoyl-CoA desaturase
- SLC27A6:
-
Solute carrier family 27, member 6
- Wnt:
-
Wingless/Int
References
Ma JS, Chang WH, Liu GH, Zhang S, Zheng AJ, Li Y, et al. Effects of flavones of sea buckthorn fruits on growth performance, carcass quality, fat deposition and Lipometabolism for broilers. Poult Sci. 2015;94(11):2641–9.
Demeure O, Duclos MJ, Bacciu N, Le Mignon G, Filangi O, Pitel F, et al. Genome-wide interval map** using SNPs identifies new QTL for growth, body composition and several physiological variables in an F2 intercross between fat and lean chicken lines. Genet Sel Evol. 2013;45:36.
Ramiah SK, Meng GY, Sheau WT, Swee KY, Ebrahimi M. Dietary conjugated linoleic acid supplementation leads to Downregulation of PPAR transcription in broiler chickens and reduction of adipocyte cellularity. PPAR Res. 2014;2014:137652.
Nishimura T. The role of intramuscular connective tissue in meat texture. Anim Sci J. 2010;81(1):21–7.
Ge K, Ye P, Yang L, Kuang J, Chen X, Geng Z. Comparison of slaughter performance, meat traits, serum lipid parameters and fat tissue between Chaohu ducks with high- and low-intramuscular fat content. Anim Biotechnol. 2020;31(3):245–55.
Frank D, Watkins P, Ball A, Krishnamurthy R, Piyasiri U, Sewell J, et al. Impact of brassica and lucerne finishing feeds and intramuscular fat on lamb eating quality and flavor. A cross-cultural study using Chinese and non-Chinese Australian consumers. J Agric Food Chem. 2016;64(36):6856–68.
Starkey CP, Geesink GH, Collins D, Hutton Oddy V, Hopkins DL. Do sarcomere length, collagen content, pH, intramuscular fat and desmin degradation explain variation in the tenderness of three ovine muscles? Meat Sci. 2016;113:51–8.
Chu W, Wei W, Han H, Gao Y, Liu K, Tian Y, et al. Muscle-specific downregulation of GR levels inhibits adipogenesis in porcine intramuscular adipocyte tissue. Sci Rep. 2017;7(1):510.
Kouba M, Bonneau M. Compared development of intermuscular and subcutaneous fat in carcass and primal cuts of growing pigs from 30 to 140kg body weight. Meat Sci. 2009;81(1):270–4.
Arrighi N, Moratal C, Clément N, Giorgetti-Peraldi S, Peraldi P, Loubat A, et al. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis. 2015;6(4):e1733.
Jiang S, Wei H, Song T, Yang Y, Peng J, Jiang S. Transcriptome comparison between porcine subcutaneous and intramuscular stromal vascular cells during adipogenic differentiation. PLoS One. 2013;8(10):e77094.
Wang S, Zhou G, Shu G, Wang L, Zhu X, Gao P, et al. Glucose utilization, lipid metabolism and BMP-Smad signaling pathway of porcine intramuscular preadipocytes compared with subcutaneous preadipocytes. Cell Physiol Biochem. 2013;31(6):981–96.
Hausman GJ, Poulos S. Recruitment and differentiation of intramuscular preadipocytes in stromal-vascular cell cultures derived from neonatal pig semitendinosus muscles. J Anim Sci. 2004;82(2):429–37.
Zhang M, Li F, Ma XF, Li WT, Jiang RR, Han RL, et al. Identification of differentially expressed genes and pathways between intramuscular and abdominal fat-derived Preadipocyte differentiation of chickens in vitro. BMC Genomics. 2019;20(1):743.
Hocquette JF, Gondret F, Baeza E, Medale F, Jurie C, Pethick DW. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal. 2010;4:303–19.
Cui HX, Guo LP, Zhao GP, Liu RR, Li QH. Method using a co-culture system with high-purity intramuscular preadipocytes and satellite cells from chicken pectoralis major muscle. Poult Sci. 2018;97(10):3691–7.
Zhou G, Wang S, Wang Z, Zhu X, Shu G, Liao W, Yu K, Gao P, ** Q, Wang XJ. Global comparison of gene expression profiles between intramuscular and subcutaneous adipocytes of neonatal landrace pig using microarray. Meat Sci. 2010;86(2):440–50.
Sun WX, Wang HH, Jiang BC, Zhao YY, **e ZR, **ong K, Chen J. Global comparison of gene expression between subcutaneous and intramuscular adipose tissue of mature Erhualian pig. Genet Mol Res. 2013;12(4):5085–101.
Mérida I, Ávila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409(1):1–18.
Sakane F, Mizuno S, Takahashi D, Sakai H. Where do substrates of diacylglycerol kinases come from Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidy-linositol turnover-independent pathways. Adv Biol Regul. 2018;67:101–8.
Lee YE, He HL, Shiue YL, Lee SW, Lin LC, Wu TF, et al. The prognostic impact of lipid biosynthesis-associated markers, HSD17B2 and HMGCS2, in rectal cancer treated with neoadjuvant concurrent chemoradiotherapy. Tumour Biol. 2015;36(10):7675–83.
Pazhouhandeh M, Sahraian MA, Siadat SD, Fateh A, Vaziri F, Tabrizi F, et al. A systems medicine approach reveals disordered immune system and lipid metabolism in multiple sclerosis patients. Clin Exp Immunol. 2018;192(1):18–32.
Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25(6):293–302.
Capobianco E, Martínez N, Fornes D, Higa R, Di Marco I, Basualdo MN, et al. PPAR activation as a regulator of lipid metabolism, nitric oxide production and lipid peroxidation in the placenta from type 2 diabetic patients. Mol Cell Endocrinol. 2013;377(1–2):7–15.
Tian W, Wang D, Wang Z, Jiang K, Li Z, Tian Y, et al. Evolution, expression profile, and regulatory characteristics of ACSL gene family in chicken (Gallus gallus). Gene. 2021;764:145094.22.
Cryer A. Tissue lipoprotein lipase activity and its action in lipoprotein metabolism. Int J BioChemiPhysics. 1981;13:525–41.
Mihelic R, Winter H, Powers JB, Das S, Lamour K, Campagna SR, et al. Genes controlling polyunsaturated fatty acid synthesis are developmentally regulated in broiler chicks. Br Poult Sci. 2020;61(5):508–17.
Wu X, Zou X, Chang Q, Zhang Y, Li Y, Zhang L, et al. The evolutionary pattern and the regulation of stearoyl-CoA desaturase genes. Biomed Res Int. 2013;2013:856521.
Hughes AL, Piontkivska H. Evolutionary diversification of the avian fatty acid-binding proteins. Gene. 2011;490(1–2):1–5.
** S, Lee SH, Lee DH, Manjula P, Lee SH, Lee JH. Genetic association of DEGS1, ELOVL6, FABP3, FABP4, FASN and SCD genes with fatty acid composition in breast and thigh muscles of Korean native chicken. Anim Genet. 2020;51(2):344–5.
Li J, **ng S, Zhao G, Zheng M, Yang X, Sun J, et al. Identification of diverse cell populations in skeletal muscles and biomarkers for intramuscular fat of chicken by single-cell RNA sequencing. BMC Genomics. 2020;21(1):752.
Liu L, Liu X, Cui H, Liu R, Zhao G, Wen J. Transcriptional insights into key genes and pathways controlling muscle lipid metabolism in broiler chickens. BMC Genomics. 2019;20(1):863.
Nafikov RA, Schoonmaker JP, Korn KT, Noack K, Garrick DJ, Koehler KJ, et al. Association of polymorphisms in solute carrier family 27, isoform A6 (SLC27A6) and fatty acid-binding protein-3 and fatty acid-binding protein-4 (FABP3 and FABP4) with fatty acid composition of bovine milk. J Dairy Sci. 2013;96(9):6007–21.
Loor JJ, Dann HM, Everts RE, Oliveira R, Green CA, Guretzky NA, et al. Temporal gene expression profiling of liver from periparturient dairy cows reveals complex adaptive mechanisms in hepatic function. Physiol Genomics. 2005;23(2):217–26.
Montserrat A, Kersten S. Regulation of lipid droplet-associated proteins by peroxisome proliferator-activated receptors. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(10 Pt B):1212–20.
He YH, He Y, Liao XL, Niu YC, Wang G, Zhao C, et al. The calcium-sensing receptor promotes adipocyte differentiation and adipogenesis through PPARγ pathway. Mol Cell Biochem. 2012;361(1–2):321–8.
Han H, Cao A, Wang L, Guo H, Zang Y, Li Z, et al. Huangqi decoction ameliorates streptozotocin-induced rat diabetic nephropathy through antioxidant and regulation of the TGF-β/MAPK/PPAR-γ signaling. Cell Physiol Biochem. 2017;42(5):1934–44.
Agarwal S, Chattopadhyay M, Mukherjee S, Dasgupta S, Mukhopadhyay S, Bhattacharya S. Fetuin-A downregulates adiponectin through Wnt-PPARγ pathway in lipid induced inflamed adipocyte. Biochim Biophys Acta Mol basis Dis. 2017;1863(1):174–81.
Cui H-X, Liu R-R, Zhao G-P, Zheng M-Q, Chen J-L, Wen J. Identification of differentially expressed genes and pathways for intramuscular fat deposition in pectoralis major tissues of fast-and slow-growing chickens. BMC Genomics. 2012;13:213.
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Map** and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621.
Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;28(1):27–30.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods Companion Methods Enzymol. 2001;25(4):402–8.
Acknowledgments
Not applicable
Funding
The research was funded by grants from the High-level talent program of Foshan University (No. cgz07243), Key-Area Research and Development Program of Guangdong Province (No. 2020B020222002), Innovation Team of Precise Animal Breeding (No. 2019KCXTD004), and Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding (No. 2019B030301010, No. 201KSYS011). The funding agencies had not involved in the experimental design, analysis, and interpretation of the data or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
ZM and NL performed the study, analyzed the data, and drafted the manuscript. JL performed the study. LL drafted the manuscript. HXC, HX, and HMK contributed to the design of the study and modifying the manuscript. GPZ and HL designed the study and was in charge of the overall project. All authors submitted comments on drafts, and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted by the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Bei**g, China). All experimental protocols were approved by the Foshan University, Animal Ethics Approval Committee under the No. 2019GA19.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
: Expression of genes (DIMFPs vs DAFPs).
Additional file 2: Table S2
: Screened known DEGs (DIMFPs vs DAFPs).
Additional file 3: Table S3
: Enriched GO terms based on DEGs (DIMFPs vs DAFPs).
Additional file 4: Table S4
: Enriched pathways based on DEGs (DIMFPs vs DAFPs).
Additional file 5: Table S5
: Screened DEGs related to lipid metabolism (DIMFPs vs DAFPs).
Additional file 6: Fig. S1
: DEGs involved in the PPAR signaling pathway, which were determined based on the 03320 pathway map in the KEGG database. The red box plot shows downregulated genes, and the green box plot shows upregulated genes in the pathway (DIMFPs vs DAFPs). The figure has obtained KEGG copyright permission.
Additional file 7: Fig. S2
: DEGs involved in the MAPK signaling pathway, which were determined based on the 04010 pathway map in the KEGG database. The red box plot shows downregulated genes, and the green box plot shows upregulated genes in the pathway (DIMFPs vs DAFPs). The figure has obtained KEGG copyright permission.
Additional file 8: Fig. S3
: DEGs involved in the calcium signaling pathway, which were determined based on the 04020 pathway map in the KEGG database. The red box plot shows downregulated genes, and the green box plot shows upregulated genes in the pathway (DIMFPs vs DAFPs). The figure has obtained KEGG copyright permission.
Additional file 9: Fig. S4
: DEGs involved in the TGF beta signaling pathway, which were determined based on the 04350 pathway map in the KEGG database. The red box plot shows downregulated genes, and the green box plot shows upregulated genes in the pathway (DIMFPs vs DAFPs). The figure has obtained KEGG copyright permission.
Additional file 10: Table S6
: Information on the specific primers used for qRT-PCR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, Z., Luo, N., Liu, L. et al. Identification of the molecular regulation of differences in lipid deposition in dedifferentiated preadipocytes from different chicken tissues. BMC Genomics 22, 232 (2021). https://doi.org/10.1186/s12864-021-07459-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-021-07459-8