Abstract
Background
Support for early detection of lung cancer has emerged from the National Lung Screening Trial (NLST), in which low-dose computed tomography (LDCT) screening reduced lung cancer mortality by 20 % relative to chest x-ray. The US Preventive Services Task Force (USPSTF) recently recommended annual screening for the high-risk population, concluding that the benefits (life years gained) outweighed harms (false positive findings, abortive biopsy/surgery, radiation exposure). In making their recommendation, the USPSTF noted that the moderate net benefit of screening was dependent on the resolution of most false-positive results without invasive procedures. Circulating biomarkers may serve as a valuable adjunctive tool to imaging.
Results
We developed a broad-based proteomics discovery program, integrating liquid chromatography/mass spectrometry (LC/MS) analyses of freshly resected lung tumor specimens (n = 13), lung cancer cell lines (n = 17), and conditioned media collected from tumor cell lines (n = 7). To enrich for biomarkers likely to be found at elevated levels in the peripheral circulation of lung cancer patients, proteins were prioritized based on predicted subcellular localization (secreted, cell-membrane associated) and differential expression in disease samples. 179 candidate biomarkers were identified. Several markers selected for further validation showed elevated levels in serum collected from subjects with stage I NSCLC (n = 94), relative to healthy smoker controls (n = 189). An 8-marker model was developed (TFPI, MDK, OPN, MMP2, TIMP1, CEA, CYFRA 21–1, SCC) which accurately distinguished subjects with lung cancer (n = 50) from high risk smokers (n = 50) in an independent validation study (AUC = 0.775).
Conclusions
Integrating biomarker discovery from multiple sample types (fresh tissue, cell lines and conditioned medium) has resulted in a diverse repertoire of candidate biomarkers. This unique collection of biomarkers may have clinical utility in lung cancer detection and diagnoses.
Similar content being viewed by others
Background
Lung cancer is the leading cause of cancer mortality in the United States. Estimates for 2014 indicate that 224,210 individuals will be diagnosed with lung cancer and 159,260 will die from the disease [1]. The average 5-year survival is about 17 %, with 79 % of cases being diagnosed as regional or distant disease. If lung cancer is detected when localized, survival increases to over 50 % [1].
Support for early lung cancer detection has emerged from the landmark NLST, where LDCT screening was shown to confer a 20 % reduction in lung cancer mortality in a high risk population [2]. Despite concerns associated with the low specificity (73.4 %) of CT screening [3] and the resulting large number of false-positive findings for lung cancer (96.4 %), the USPSTF recently recommended annual LDCT-screening for lung cancer in high-risk individuals [4, 5]. In their recommendation statement, the USPSTF stressed the need for more research into the use of biomarkers to complement LDCT screening. Two key clinical opportunities exist. First, the use of biomarkers for early detection of lung cancer could define a new high-risk population or refine the screening criteria recommended by USPSTF (age: 55 to 80 years, smoking history: >30 pack-year). Such biomarkers would serve as a pre-imaging filter, reducing the overall cost of screening and lowering the number of false-positive findings and unnecessary follow-up procedures. The second opportunity lies in improving the accuracy of lung cancer diagnosis. Given the high frequency of positive findings (pulmonary nodules) with CT screening [2], new means of accurately determining malignant risk are urgently required. In the NLST, 24 % of surgically resected nodules were found to be benign [2]. By improving the accuracy with which malignant risk is determined, biomarkers could potentially enhance diagnostic management by reducing unnecessary surgical intervention, minimizing the use of costly PET-CT and lowering radiation exposure associated with CT monitoring, while enabling detection of lung cancer at an early, more curable, stage.
A wide variety of approaches have been utilized to discover new blood-based lung cancer protein biomarkers [6]. These range from splice variant analysis and the isolation of tumor-enriched transcripts [7], to the development of novel proteomic platforms with the capacity to resolve candidate markers in a highly multiplexed fashion [8]. Advances in mass spectrometry (MS)-based technologies have also enabled discovery of new lung cancer biomarker candidates directly in serum or plasma [9–13]. While the identification of biomarkers directly in blood-based matrices can be problematic due to their complexity and the presence of multiple highly abundant factors [14], some of these challenges can be minimized through extensive fractionation [15]. Differentially expressed candidate markers have also been successfully identified through comparison of blood draining from the tumor vascular bed matched with systemic arterial blood from the same patient [
The Panther based classification system was used to categorize markers based on Protein Class and Pathway [22, 23]. Protein classes were defined for 141/179 (79 %) of the candidate markers evaluated. The most common classes reported were: receptors (14 %), cell adhesion molecules (14 %), hydrolases (13 %), defense/immunity proteins (10 %), proteases (9 %), enzyme modulators (8 %) and signaling molecules (8 %; Additional file 4: Figure S1). Further protein class analysis revealed similar profiles for biomarkers identified in the two cell-surface based discovery programs, resected tissue and cultured lung cancer cell lines (Additional file 5: Figure S2). Panther classification resolved protein categories for 91/113 (81 %) of the markers identified in tissues and 70/86 (81 %) of those found in cell lines. While some differences clearly exist, the most abundant protein classes (cell adhesion, defense/immunity, enzyme modulator, extracellular matrix, hydrolase, protease, receptor, signaling, transfer/carrier and transporter) were resolved in both tissues and cell lines. Panther-based pathway analysis also revealed many similarities between the two discovery platforms. Pathways commonly identified in resected tissues (integrin signaling, inflammation, gonadotropin releasing hormone receptor, Alzheimer disease-presenilin and plasminogen activating cascade) were also frequently found in the cell lines studied. Some differences were resolved between the two sources, including enrichment of blood-coagulation related proteins in the tissue based discovery system (22 %) relative to cell line studies (9 %; Additional file 6: Figure S3).
Serum-based biomarker verification
ELISA analysis was undertaken to investigate whether the differential expression profiles observed in lung cancer tissues, cell lines and conditioned medium, would also be detected in the bloodstream of subjects with lung cancer. A small number of candidates were selected for serological characterization: CEA, MDK, MMP2, SLPI, TFPI and TIMP1 (Table 1). These biomarkers were selected in part due to the reagent availability, but also, with the exception of CEA, because they represented some of the more novel lung cancer markers identified, with few studies indicating elevated expression in the circulation of patients with early stage disease [24]. While all six markers had been shown to be present in plasma [25], they had not been resolved in other proteomic studies aimed at identifying differentially expressed lung cancer markers using alternative biological fluids: bronchial lavage [26, 27] sputum [28] or pleural fluid [29, 30], or in profiling experiments aimed at identifying markers associated with other common lung disorders: COPD [27], asthma [31] or tuberculosis [32].
With the goal of identifying markers to be used to screen for early-stage disease, or to guide diagnosis following CT-based detection, expression levels were determined in subjects with stage I NSCLC (n = 94), relative to normal smoker controls (n = 189; Table 2). In an effort to minimize selection of markers associated with pre-analytical variability, where differential expression profiles may be derived from serum sample collection procedures specific to any single clinical study site, subjects from two independent clinical studies were combined into a single testing set. The first study collected at CRCCC (Clinical Research Center of Cape Cod; West Yarmouth, MA), comprised patients with stage I NSCLC (n = 30) and healthy smoker controls (n = 99). The second cohort, collected at New York University (NYU) School of Medicine/Langone Medical Center, was selected from a high-risk population with a history of heavy tobacco usage. Serum samples were collected from patients with stage I NSCLC (n = 64) and healthy controls (n = 90).
Levels of five of the six candidate biomarkers tested (CEA, MDK, MMP2, SLPI, TIMP1, TFPI) were significantly higher in serum from subjects with NSCLC than in controls (Table 3, Additional file 7: Figure S4), serving to support this indirect discovery approach. Three extensively characterized markers: CYFRA 21–1, SCC and OPN were also evaluated. These markers served as a reference in evaluating clinical accuracy of the MS-identified markers.
Multi-marker model development and testing
The identification of multiple differentially expressed markers prompted the development of a multi-marker panel. Elastic net modeling [33] started with all 9 candidate markers (Table 3). The optimal value of the regularization parameter, as determined by bootstrap resampling, reduced the parameter estimate for SLPI to zero, while the remaining 8 markers: TFPI, MDK, OPN, MMP2, TIMP1, CEA, CYFRA 21–1 and SCC, which retained non-zero coefficients, were selected in the final model. In the training dataset (Table 2), this 8-marker model resolved lung cancer patients from smoker controls with 75 % sensitivity at 90 % specificity (AUC = 0.913). A bootstrap validation procedure confirmed clinical performance of the model, AUC = 0.903.
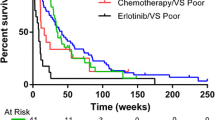
The accuracy of the 8-marker model was tested in an independent study (Mayo Clinic). Controls (n = 50) were selected from the high risk control population evaluated in the Mayo CT-Screening Trial [34] and included subjects with pulmonary nodules (n = 22). Lung cancer cases were pre-operative surgical referrals (n = 50). Malignant lesions were significantly larger than screen detected benign nodules. Cases and controls were matched on age, gender and smoking history (Table 2). EDTA plasma samples were utilized in this study. Levels of all markers included in the model had been shown to be highly correlated in serum and EDTA plasma (Additional file 8: Table S4). The 8-marker model distinguished patients with malignant lesions from all smoker controls with an AUC = 0.775 (Fig. 2), accurately classifying control subjects with (AUC = 0.745) or without pulmonary nodules (AUC = 0.799).
While the 8-marker model was found to be substantially correlated with nodule size (r = 0.739; p < 0.0001), it was not associated with any of the other clinicopathological variables tested: age, sex, smoking history (unpublished data). Elevated expression of the multi-marker model was observed in tumors with a squamous cell histology, relative to adenocarcinoma cases (p = 0.019), driven in part by higher levels of CYFRA 21-1 (p < 0.0001) and OPN (p = 0.013) in squamous cell carcinomas (unpublished data).
Discussion
LDCT screening of high-risk smokers has been shown to reduce lung cancer mortality by 20 %, relative to chest radiography. However, of the 24.2 % of participants with an abnormal screening test, the vast majority (96.4 %) were false positives for lung cancer. The low positive predictive value of LDCT results in (i) higher screening costs and (ii) unnecessary invasive procedures for benign disease. Non-invasive biomarkers are urgently needed to improve LDCT-based screening. Biomarkers could be used to refine the high-risk population, thus limiting the number of individuals being screened by LDCT. Alternatively, biomarkers could be employed following screening, to distinguish relatively rare malignant nodules from commonly found benign nodules. A number of novel blood-based markers have recently been characterized [7, 35], some of which have been evaluated in the form of multi-marker panels [15, 36–38]. However, to date, very few have been shown to add value to clinical variables already being employed in evaluating malignant risk [39].
Marker discovery in blood-based systems (serum and plasma) has been hampered by the complexity of these matrices and presence of multiple highly abundant proteins. Alternative “indirect” approaches have successfully been applied to: freshly resected clinical specimens, primary cultures, cell lines cultured in vitro and in vivo and conditioned medium, with collections of candidate biomarkers identified in each. However, as each of these studies has been performed in isolation, it has been difficult to evaluate the relative merits of each of these approaches. We report, for the first time, a discovery approach that combines multiple cellular systems: resected tissues, cultured cell lines and conditioned medium. In so doing, we have identified a number of markers commonly resolved across the platforms (Fig. 1). It is noteworthy that while significant overlap across the systems clearly exists, with similar signaling pathways apparently activated across the different discovery systems (Additional file 6: Figure S3), the majority of candidate markers were identified in only one of the three programs. Integrating discoveries from the three systems has not only served as a starting point to understand the relative merits of these distinct approaches, but has also produced a diverse pool of candidate markers for future validation.
All 179 candidate markers selected exhibited at least a four-fold increased level of expression in lung cancer samples relative to appropriate controls. While this 4.0X cut point provided a simple means of identifying the most differentially expressed biomarkers, additional approaches using different cut-points and possibly integrating key clinical variables such as histology and stage, will likely reveal a more extensive collection of candidates for future studies.
Analyses of the glycoproteins residing at the cell surface, in both tissues and cell lines, enabled discovery of cell-membrane markers that may be shed and released into the peripheral circulation. CEA (CEACAM5) provides an example of this type of cell-surface marker, as it is shed into the bloodstream and detected at elevated levels in a wide variety of malignant disorders [40]. While the molecular mechanism responsible for shedding remains unclear [41], CEA is a widely employed serum biomarker used in prognosis, staging and monitoring of colorectal cancer. In addition to CEA, several other cell-surface markers known to be shed into the circulation were identified in these studies including: MET (c-Met proto-oncogene product, hepatocyte growth factor receptor) [42], mesothelin [43], EPCAM [44], and ICAM-1 [45]. It is noteworthy that many additional cell-membrane markers, with similar secondary structures, were also resolved (Additional file 3: Table S3) and may serve as a valuable pool of candidate markers for future studies.
While CEA (CEACAM5) represents a well-characterized tumor biomarker, the association of other biomarkers with lung cancer varies considerably, with limited evidence of differential expression in early-stage disease. Increased activity of TFPI, a Kunitz-type serine protease inhibitor, has been reported in the circulation of patients with late-stage NSCLC [46]. MDK appears to play a role in both angiogenesis [47] and lung cancer metastasis [48]. Elevated levels of MDK, a heparin-binding growth factor, have been observed in serum collected from patients with a broad range of solid tumors, including lung cancer [49]. A number of tumor-stimulating functions have been demonstrated for TIMP1 [50, 51]. Elevated levels of this metallopeptidase have been observed in serum collected from subjects with late-stage NSCLC [52], with the highest levels reported in squamous cell carcinoma [53]. SLPI, a member of the Kazal superfamily of serine-proteinases, appears to play a role in tumor growth and metastasis [54–57]. Elevated levels of SLPI protein have been observed in the bloodstream of patients with NSCLC [24, 58]. While the matrix metalloproteinase MMP2 appears to play a role in lung cancer growth and migration [59–61], studies investigating levels of MMP2 in the bloodstream have reported inconsistent findings [62–64]. The diverse range of biological functions observed for markers identified in these studies are summarized in Table 4.
Our ELISA-based serological studies evaluated six candidate markers identified in these LC/MS analyses, along with three additional markers (CYFRA 21-1, SCC and OPN) that served as a benchmark of clinical accuracy. Two of these markers, CYFRA 21–1 and SCC, would not have been predicted to be resolved in these LC/MS studies as they lack an N-linked glycosylation site required for selection in the glycoprotein enrichment procedure. In contrast, two N-linked glycosylation sites are present in the mature form of the secreted protein OPN; as such, we would have expected peptides derived from this marker to be identified in these studies. It is unclear why OPN was not resolved; however it is possible that this marker may not have been differentially expressed in the samples analyzed, or that OPN-derived peptides may have been masked in the LC/MS separation.
The ability of the multi-marker model to distinguish lung cancer cases from control subjects with or without nodules indicates potential roles for the test, either as an adjunct to CT- screening, in determining risk of malignancy of pulmonary nodules, or in early lung cancer screening. Clearly additional studies are required to better characterize clinical performance of the current model and to evaluate of larger numbers of candidate biomarkers revealed in this study.
Conclusions
Given the low PPV (4 %) of LDCT in screening the high risk population, there is a pressing need to discover non-invasive biomarkers to complement radiographic imaging in lung cancer screening and diagnosis.
We describe a broad-based discovery platform that has enabled the identification of a large, diverse collection of candidate lung cancer biomarkers. A subset of these markers identified “indirectly” in freshly resected tissue, cell lines and conditioned medium retained elevated cancer-associated expression profiles in the circulation of patients with early-stage disease. A multi-marker model was developed which accurately distinguished lung cancer cases from high risk smokers. This unique collection of markers should serve as a valuable resource for future clinical validation studies.
Methods
Tissue specimens
Freshly resected lung specimens (malignant lesions and normal adjacent tissue) were collected from 4 clinical sites using IRB-approved protocols: 1. Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA; 2. Division of Thoracic Surgery, University of Maryland Medical Center, Baltimore, MD; 3. Department of Cardiothoracic Surgery, George Washington University, Washington DC; 4. Asterand, Detroit, MI. To enrich for samples likely to produce strong MS signal, tissue specimens with a mass of at least 1 g were selected for this study. Single cell suspensions were prepared from each resected sample using a standard methodology before removal of red blood cells through addition of ACK lysis buffer [65]. Epithelial (EpCAM), leukocyte (CD45) content and cellular viability (PI exclusion) were determined by flow cytometry analysis (LSR I, BD Biosciences, San Jose, CA). Epithelial enrichment was undertaken using flow cytometry based cell sorting (EpCAM, Clone EBA-1, BD Biosciences). Samples yielding a minimum of 1x106 viable epithelial cells were submitted for MS analysis.
Cell lines and tissue culture
Lung cancer cell lines obtained from American Type Culture Collection (ATCC, Manassas, VA) or European Collection of Cell Cultures (ECACC, Salisbury, UK) were cultured in the appropriate media as recommended.
Conditioned medium
Cell-lines were cultured to 70 % confluence, transferred to protein free media (293 SFM II, Invitrogen, Carlsbad, CA) for 72 h, after which cell debris was removed by centrifugation.
Blood-based studies
Serum: A total of 283 subjects were evaluated in the verification/model training study, healthy smoker controls n = 189 and early stage NSCLC cases n = 94 (Table 2). Samples were collected during the period 2003–2008. Histological classification followed WHO guidelines recommended at the time of diagnosis.
Plasma: EDTA plasma was collected from 100 subjects in the model testing study, controls n = 50 and cases n = 50 (Table 2).
Serum: plasma correlations: Blood was drawn from subjects (n = 10) and collected into serum (red-cap) and EDTA plasma tubes on the same visit to the clinic (CRCCC). Concentrations levels were determined for all candidate biomarkers (n = 9). Marker levels were highly correlated (Additional file 8: Table S4).
For all studies, written informed consent was obtained from each subject. Samples were obtained prior to any treatment and were stored at −80 °C until use.
Mass spectrometry
The discovery approach combined the enrichment of cell surface glycoproteins and secreted proteins with a decoupled (label-free), quantitative proteomics method. These programs focused discovery on markers containing short, tryptically-cleaved 5–25 amino acid peptides encoding either a cysteine residue or an N-linked glycosylation site, providing broad coverage of the proteome. A quantitative liquid LC/MS analysis of normal and tumor samples was used to identify peptide ions that were expressed at >4x levels in the cancer cells relative to the adjacent normal tissue. In cell line studies tumor lines were compared to Beas-2B. Subsequent MS/MS identification focused exclusively on peptides that had a relative change in abundance. To ensure data quality, manual inspection of each differentially expressed peptide ion was performed.
Cell surface protein enrichment
Viable cells were incubated with 1 mM sodium periodate for 10 min to oxidize glycoproteins [66]. Oxidized glycoproteins were conjugated to hydrazide resin (Bio-Rad, Hercules, CA) at 4 °C overnight [67]. After washing sequentially with: 2 M NaCl, 2 % SDS, 200 mM propanolamine (0.1 M NaAcetate, pH 5.5), 40 % ethanol and 80 % ethanol; bound proteins were reduced with dithiothreitol, and alkylated with ICAT™ reagent (Life Technologies/Thermo Fisher Scientific, Applied Biosystems, Framingham, MA). Alkylated proteins were digested with trypsin and cysteine-containing peptides were captured using an avidin column (Life Technologies/Thermo Fisher Scientific). In addition to the cysteine-containing peptide fraction, peptides bound to the resin were also collected and analyzed. Release of peptides was achieved through PNGase-F digestion (New England BioLabs, Ipswich, MA.). While we found some overlap between the proteins identified in the two fractions, analysis of both the cysteine -containing fraction and the resin-bound fraction resulted in complementary coverage of the cell surface protein population.
Conditioned medium preparation
Samples were lyophilized, reconstituted with deionized H2O, and dialyzed against 0.6 M Guanidine HCl, 10 mM Tris buffer, pH 8. Proteins were reduced with Tris (2-carboxyethyl) phosphine and alkylated with ICAT™ reagent (Life Technologies/Thermo Fisher Scientific). Following dialysis (0.1 M NH4Acetate), alkylated proteins were digested with trypsin. Cysteine-containing peptides were purified using an avidin column (Life Technologies/ Thermo Fisher Scientific).
LC/MS analysis
Peptides, including standards used for mass calibration and retention time normalization, were separated and analyzed using methods of Kim et al. [68].
Data alignment and expression analysis
Peptide ion peaks of LC/MS maps were aligned based on mass to charge ratio (m/z), retention time (Rt), and charge state (z). Retention time normalization was accomplished in two steps: a primary alignment using the internal standard peptides and a secondary fine tuning using all of the common features. Ion intensities were normalized across normal and tumor samples by minimizing the sum of the differences between the intensities of each of the ions and the mean intensity for that ion across all maps. Differentially expressed peptide ions were manually verified before LC-MS/MS-based peptide sequencing. Subcellular predictions determined by UniProt, release 2014_07 [69].
Serum/plasma analyses
Enzyme-linked immunosorbent assay (ELISA) kits were obtained from a variety of commercial sources: Bio-Techne/R&D Systems, Minneapolis, MN (MMP2, OPN, SLPI, TFPI); Siemens Healthcare Diagnostics, Cambridge, MA (TIMP1); and IBL-America, Minneapolis, MN (CEA, CYFRA 21–1, MDK, SCC). Assays were performed following the manufacturers’ instructions. Plates were read on a Spectra Max M2 Microplate Reader (Molecular Devices, Sunnyvale, CA.).
Model development
Logistic regression of lung cancer status on the 9 candidate markers (ng/mL) via elastic net regularization was employed to select a final set of markers and their associated parameter estimates. Elastic net regularization penalizes the parameter estimates (shrinks them toward zero) and performs variable subset selection by allowing sufficiently small parameter estimates to be reduced entirely to zero. Regularization of the parameter estimates tends to produce stable regression models with smaller prediction error than those that are not regularized. Bootstrap resampling (10,000 iterations), and maximization of the mean area under the ROC curve (AUC) for the out-of-bag samples, was used to select the optimal weight of the shrinkage penalty.