Abstract
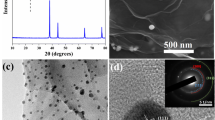
Energy is the basic need of world, and in this modern era to achieve global need, electrochemical water splitting knwon as process through which world can fulfill their energy requirements, but an efficient and affordable electrocatalyst is necessary for this process. Here, Ag2S/rGO nanocomposite was prepared using hydrothermal route as electrocatalyst for this (OER) process. The hydrothermal method leads to the development of nanocrystals having larger surface area and excellent morphology. The prepared nanocomposite undergoes physical and electrochemical testing. The physical characterization confirmed that Ag2S/rGO has a dynamic surface area (790.6 cm2), providing more active sites; hence, more extensive electron transformation occurs. The electrochemical testing in an alkaline medium with three electrodes was used to know about electrocatalytic action of the prepared nanocomposite. The prepared nanocrystal achieved overpotential 179.56 mV, Tafel slope (31.6 mV dec−1) and TOF (1.40 s−1) with high durability. All these finding confirmed that the fabricated Ag2S/rGO is a strong electrocatalyst for OER.









Similar content being viewed by others
Data availability statements
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The manuscript has associated data in a data repository.
References
M. Lahieb Faisal et al., The effect of smoke from factories, electricity generator and vehicles on human health and environment. A review. Solid State Technol. 63(6), 21390–21398 (2020)
J.S. Riti, Y. Shu, Renewable energy, energy efficiency, and eco-friendly environment (R-E5) in Nigeria. Energy Sustain. Soc. 6(1), 1–16 (2016)
X. Han et al., Multifunctional TiO2/C nanosheets derived from 3D metal–organic frameworks for mild-temperature-photothermal-sonodynamic-chemodynamic therapy under photoacoustic image guidance. J. Colloid Interface Sci. 621, 360–373 (2022)
F. Martins et al., Analysis of fossil fuel energy consumption and environmental impacts in European countries. Energies 12(6), 964 (2019)
T.-Z. Ang et al., A comprehensive study of renewable energy sources: classifications, challenges and suggestions. Energy Strategy Rev. 43, 100939 (2022)
Y. Zheng et al., Sulfur-doped g-C3N4/rGO porous nanosheets for highly efficient photocatalytic degradation of refractory contaminants. J. Mater. Sci. Technol. 41, 117–126 (2020)
L. Chen et al., Reinforced AZ91D magnesium alloy with thixomolding process facilitated dispersion of graphene nanoplatelets and enhanced interfacial interactions. Mater. Sci. Eng. A 804, 140793 (2021)
N. Wang et al., Acetate ions facilitated immobilization of highly dispersed transition metal oxide nanoclusters in mesoporous silica. Inorg. Chem. (2024)
G. Zhao, et al., Understanding the role of transition metal single-atom electronic structure in oxysulfur radical-mediated oxidative degradation. Environ. Sci. Ecotechnol. 100405 (2024)
L.-Y. Tian, et al., Metal-organic frameworks based on ternary transition metal ions for high-performance lithium ion batteries. J. Solid State Chem. 124717 (2024)
S. Manzoor et al., Development of ZnCo alloy enclosed in N-doped carbon with hexagonal close packing crystal phase inspires potential oxygen evolution reaction. J. Alloy. Compd. 924, 166439 (2022)
A.A. Alothman, et al., Facile fabrication of CuScS2/CoO as an efficient electrocatalyst for oxygen evolution reaction and water treatment process. Int. J. Hydrog. Energy (2023)
T. Reier, M. Oezaslan, P. Strasser, Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. ACS Catal. 2(8), 1765–1772 (2012)
X. Li et al., Nanostructured catalysts for electrochemical water splitting: current state and prospects. J. Mater. Chem. A 4(31), 11973–12000 (2016)
K.C. Majhi, M. Yadav, Bimetallic chalcogenide nanocrystallites as efficient electrocatalyst for overall water splitting. J. Alloy. Compd. 852, 156736 (2021)
M. Liu et al., Mechanism of electrocatalytic CO2 reduction reaction by borophene supported bimetallic catalysts. J. Colloid Interface Sci. 659, 959–973 (2024)
S. **ng, Nanomaterials of conducting polymers and its application in energy conversion and storage, in Advanced Nanomaterials for Electrochemical-Based Energy Conversion and Storage. (Elsevier, 2020), pp.325–354
X. Lu et al., One-dimensional conducting polymer nanocomposites: synthesis, properties and applications. Prog. Polym. Sci. 36(5), 671–712 (2011)
Y. Liu et al., Research progress of oxygen evolution reaction catalysts for electrochemical water splitting. Chemsuschem 14(24), 5359–5383 (2021)
W. Li, C. Wang, X. Lu, Integrated transition metal and compounds with carbon nanomaterials for electrochemical water splitting. J. Mater. Chem. A 9(7), 3786–3827 (2021)
M. Itagi, D. Chauhan, Y.-H. Ahn, HfCoS/rGO bifunctional electrocatalysts for efficient water splitting in alkaline media. Energy Fuels 37(15), 11298–11308 (2023)
Y.-R. Hong et al., Synthesis of transition metal sulfide and reduced graphene oxide hybrids as efficient electrocatalysts for oxygen evolution reactions. R. Soc. Open Sci. 5(9), 180927 (2018)
M. Zhao et al., One step hydrothermal synthesis of Ni-MoS2-RGO bifunctional electrocatalysts for HER and OER. Int. J. Electrochem. Sci. 16(3), 210323 (2021)
C. Shuai et al., Hierarchical NiCo2S4 nanosheets grown on graphene to catalyze the oxygen evolution reaction. J. Mater. Sci. 55(4), 1627–1636 (2020)
J. Jiang et al., Ultrahigh electrocatalytic oxygen evolution by iron-nickel sulfide nanosheets/reduced graphene oxide nanohybrids with an optimized autoxidation process. Nano Energy 43, 300–309 (2018)
Y. Guo et al., Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting. Adv. Mater. 31(17), 1807134 (2019)
R. Souleymen et al., Microwave-assisted synthesis of graphene-like cobalt sulfide freestanding sheets as an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 6(17), 7592–7607 (2018)
A.G. Abid et al., Facile synthesis of scheelite-type NdOsO4 directly grown on carbon cloth for oxygen evolution reaction. J. Solid State Electrochem. 26(11), 2401–2410 (2022)
A.V. Rane et al., Methods for synthesis of nanoparticles and fabrication of nanocomposites, in Synthesis of Inorganic Nanomaterials. (Elsevier, 2018), pp.121–139
K. Byrappa, T. Adschiri, Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 53(2), 117–166 (2007)
Y. Guo et al., One pot preparation of reduced graphene oxide (RGO) or Au (Ag) nanoparticle-RGO hybrids using chitosan as a reducing and stabilizing agent and their use in methanol electrooxidation. Carbon 50(7), 2513–2523 (2012)
I. Boukhoubza, et al. X-ray diffraction investigations of nanostructured ZnO coated with reduced graphene oxide. J. Phys. Conf. Ser. (2019)
K.J. Babu, K.S. Nahm, Y.J. Hwang, A facile one-pot green synthesis of reduced graphene oxide and its composites for non-enzymatic hydrogen peroxide sensor applications. RSC Adv. 4(16), 7944–7951 (2014)
Y. Ma et al., In-situ synthesis strategy of monodispersed Ag2S nanoparticles to modify wear resistance of polyamide-imide nanocomposite lubricating coatings. Tribol. Lett. 67, 1–11 (2019)
R. Zamiri et al., The structural and optical constants of Ag2S semiconductor nanostructure in the Far-Infrared. Chem. Cent. J. 9(1), 1–6 (2015)
R.A. Rochman, et al. Preparation of nitrogen and sulphur Co-doped reduced graphene oxide (rGO-NS) using N and S heteroatom of thiourea. In IOP Conference Series: Materials Science and Engineering (IOP Publishing, 2019).
D. Kichukova et al., Facile synthesized Cu–RGO and Ag–RGO nanocomposites with potential biomedical applications. Nanomaterials 12(12), 2096 (2022)
P.J. Mafa et al., Visible light driven ZnMoO4/BiFeWO6/rGO Z-scheme photocatalyst for the degradation of anthraquinonic dye. J. Phys. Chem. C 123(33), 20605–20616 (2019)
Inamuddin, N. Shakeel, Optimization of rGO-PEI/Naph-SH/AgNWs/Frt/GOx nanocomposite anode for biofuel cell applications. Sci. Rep. 10(1), 8919 (2020)
L. Kashinath, K. Namratha, K. Byrappa, Sol-gel assisted hydrothermal synthesis and characterization of hybrid ZnS-RGO nanocomposite for efficient photodegradation of dyes. J. Alloy. Compd. 695, 799–809 (2017)
L. Han, S. Dong, E. Wang, Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv. Mater. 28(42), 9266–9291 (2016)
L. Zheng et al., Photo/electrochemical applications of metal sulfide/TiO2 heterostructures. Adv. Energy Mater. 10(1), 1902355 (2020)
A. Arulraj et al., Direct synthesis of cubic shaped Ag2S on Ni mesh as binder-free electrodes for energy storage applications. Sci. Rep. 9(1), 10108 (2019)
J. Zhang et al., A highly conductive porous graphene electrode prepared via in situ reduction of graphene oxide using Cu nanoparticles for the fabrication of high performance supercapacitors. RSC Adv. 5(67), 54275–54282 (2015)
Q. Liu et al., A multijunction of ZnIn2S4 nanosheet/TiO2 film/Si nanowire for significant performance enhancement of water splitting. Nano Res. 8, 3524–3534 (2015)
M. Sadaqat et al., Iron doped nickel ditelluride hierarchical nanoflakes arrays directly grown on nickel foam as robust electrodes for oxygen evolution reaction. Electrochim. Acta 371, 137830 (2021)
D.A. Alshammari et al., Tuning the electrocatalytic efficacy of nano-dumbbell shaped nickel selenide anchored cobalt telluride towards oxygen evolution. J. Electroanal. Chem. 945, 117701 (2023)
S. Sultan et al., Single atoms and clusters based nanomaterials for hydrogen evolution, oxygen evolution reactions, and full water splitting. Adv. Energy Mater. 9(22), 1900624 (2019)
S. Choi et al., Reduced graphene oxide-based materials for electrochemical energy conversion reactions. Carbon Energy 1(1), 85–108 (2019)
M.Y. ur Rehman et al., Facile synthesis of novel carbon dots@ metal organic framework composite for remarkable and highly sustained oxygen evolution reaction. J. Alloy. Compd. 856, 158038 (2021)
C.A. Carrero et al., Anomalous reactivity of supported V2 O5 nanoparticles for propane oxidative dehydrogenation: influence of the vanadium oxide precursor. Dalton Trans. 42(35), 12644–12653 (2013)
S. Aman et al., Facile synthesis of CuZrO3@ PPY nanohybrid balls embedded 3-dimensional network with synergistic effect for efficient oxygen evolution reaction. Surf. Interfaces 36, 102607 (2023)
D. Shao et al., One-step preparation of Fe-doped Ni3S2/rGO@ NF electrode and its superior OER performances. Int. J. Hydrog. Energy 44(5), 2664–2674 (2019)
A. Rebekah et al., Effect of cation substitution in MnCo2O4 spinel anchored over rGO for enhancing the electrocatalytic activity towards oxygen evolution reaction (OER). Int. J. Hydrog. Energy 45(11), 6391–6403 (2020)
P. Shinde et al., Optimized performance of nickel in crystal-layered arrangement of NiFe2O4/rGO hybrid for high-performance oxygen evolution reaction. Int. J. Hydrog. Energy 46(2), 2617–2629 (2021)
S.S. Narwade et al., Ni/NiO@ rGO as an efficient bifunctional electrocatalyst for enhanced overall water splitting reactions. Int. J. Hydrog. Energy 44(49), 27001–27009 (2019)
Y. Gu et al., Electronic structure tuning in Ni3FeN/r-GO aerogel toward bifunctional electrocatalyst for overall water splitting. ACS Nano 12(1), 245–253 (2018)
Y. Guo et al., Mesoporous iron-doped MoS2/CoMo2S4 heterostructures through organic–metal cooperative interactions on spherical micelles for electrochemical water splitting. ACS Nano 14(4), 4141–4152 (2020)
J. Kundu et al., Ni-Doped CuS as an efficient electrocatalyst for the oxygen evolution reaction. Catal. Sci. Technol. 9(2), 406–417 (2019)
J. Zhu et al., Study of active sites on Se-MnS/NiS heterojunctions as highly efficient bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 7(47), 26975–26983 (2019)
W.-H. Huang et al., CeO2-embedded mesoporous CoS/MoS2 as highly efficient and robust oxygen evolution electrocatalyst. Chem. Eng. J. 420, 127595 (2021)
P. Babar et al., SILAR deposited iron phosphate as a bifunctional electrocatalyst for efficient water splitting. J. Colloid Interface Sci. 534, 350–356 (2019)
J. Masa, W. Schuhmann, The role of non-metallic and metalloid elements on the electrocatalytic activity of cobalt and nickel catalysts for the oxygen evolution reaction. ChemCatChem 11(24), 5842–5854 (2019)
M. Sadaqat et al., Zinc-telluride nanospheres as an efficient water oxidation electrocatalyst displaying a low overpotential for oxygen evolution. J. Mater. Chem. A 7(46), 26410–26420 (2019)
N. Nazar et al., Metal-organic framework derived CeO2/C nanorod arrays directly grown on nickel foam as a highly efficient electrocatalyst for OER. Fuel 307, 121823 (2022)
Acknowledgements
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project (Grant No. PNURSP2024R378), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The Deanship of Research and Graduate Studies at King Khalid University is greatly appreciated for funding this work through Large Research Project under grant number RGP2/32/45. A.M.A. Henaish thanks the the Ministry of Science and Higher Education of the Russian Federation (Ural Federal University Program of Development within the Priority-2030 Program) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
All the author have done equal work.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest by any form for this manuscript. The authors declare that they have no conflict of interest.
Ethical approval
Yes this article compliance with ethical standards of journal.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nazim, M., Alrowaily, A.W., Alotaibi, B.M. et al. Ag2S nanoflakes decorated over rGO nanosheets: a sustainable and highly efficient electrocatalyst for oxygen evolution reaction. Eur. Phys. J. Plus 139, 528 (2024). https://doi.org/10.1140/epjp/s13360-024-05285-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-024-05285-x