Abstract
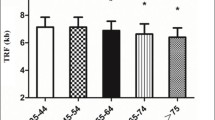
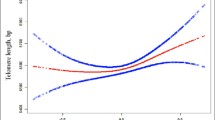
Decreased renal function is diagnosed in a great number of people aged over 60. Decreased glomerular filtrationrate varies widely within different age ranges. One of the probable mechanisms associated with the steeper decline of renal function may be a shortening in telomere length due to some chronic inflammation. The objective of this research was to study the association of renal function with telomere length and the indicators of chronic inflammation in patients without chronic kidney disease and cardiovascular diseases. The study involved 253 patients (81 men and 172 women) with the mean age of 51.5 ± 13.3 years without chronic kidney disease and cardiovascular diseases. Of the participants, 55 patients had hypertension of 1‒2 degree, 46 patients had normal parameters of renal function, and 207 participants were characterized by a mild failure in renal function. The level of albuminuria in all patients was below 30 mg/24 h. A multivariate linear regression analysis, with consideration of age- and gender-related differences, has shown a statistically significant association of albuminuria levels with telomere lengths (p = 0.023), CRP (p = 0.047), and fibrinogen (p = 0.001). No associations have been found between telomere length and inflammatory markers, on the one hand, and the levels of glomerular filtration rate, urea and creatinine, on the other hand, although the latter well correlated with age, (p < 0.001). It has been shown that albuminuria is associated with chronic inflammation and telomere length (the marker of replicative cell senescence) to a larger extent than all other renal function indicators under study. Albuminuria can be regarded as the principal target for a therapeutic approach to prevent changes in renal function and the vascular wall.
Similar content being viewed by others
References
Bikbov, B.T. and Tomilina, N.A., The status of substitutive therapy of patients with chronic renal failure in Russian Federation in 1998–2007: analytical report according to the results of Russian inventory on substitutive renal therapy, Nefrol. Dializ, 2009, no. 11 (3), pp. 144–233.
Agewall, S., Fagerberg, B., Attvall, S., et al., Microalbuminuria, insulin sensitivity and haemostatic factors in non-diabetic treated hypertensive men: risk factor intervention study group, J. Int. Med., 1995, vol. 237, pp. 195–203.
Anderson, S. and Brenner, B.M., Effects of aging on the renal glomerulus, Am. J. Med., 1986, vol. 80, pp. 435–442.
Festa, A., D’agostino, R., Howard, G., et al., Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: the insulin resistance atherosclerosis study, Kidney Int., 2000, vol. 58, pp. 1703–1710.
Bansal, N., Whooley, M.A., Regan, M., et al., Association between kidney function and telomere length: the heart and soul study, Am. J. Nephrol., 2012, vol. 36, no. 5, pp. 405–411.
Berton, G., Citro, T., Palmieri, R., et al., Albumin excretion rate increases during acute myocardial infarction and strongly predicts early mortality, Circulation, 1997, vol. 96, pp. 3338–3345.
Blasco, M.A., Telomeres and human disease: ageing, cancer and beyond, Nat. Rev. Genet., 2005, vol. 6, pp. 611–622.
Bonventre J.V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure, J. Am. Soc. Nephrol., 2003, vol. 14, suppl. 1, pp. 55–61.
Boxall, M.C., Goodship, T.H., Brown, A.L., et al., Telomere shortening and haemodialysis, Blood Purif., 2006, vol. 24, pp. 185–189.
Csiszar, A., Toth, J., Peti-Peterdi, J., et al., The aging kidney: role of endothelial oxidative stress and inflammation, Acta Physiol. Hung., 2007, vol. 94, pp. 107–115.
Fehrman-Ekholm, I. and Skeppholm, L., Renal function in the elderly >70 years old) measured by means of lohexol clearance, serum creatinine, serum urea and estimated clearance, Scand. J. Urol. Nephrol., 2004, vol. 38, no. 1, pp. 73–77.
Glassock, R.J. and Winearls, C., Ageing and the glomerular filtration rate: truths and consequences, Trans. Am. Clin. Climatol. Ass., 2009, vol. 120, pp. 419–428.
Goslin, P., Sutcliffe, A.J., Cooper, M.A., and Jones, A.F., Burn and trauma associated proteinuria: the role of lipid peroxidation, rennin, and myoglobin, Ann. Clin. Biochem., 1988, vol. 25, pp. 53–59.
Gosling, P., Shearman, C.P., Gwynn, B.R., et al., Microproteinuria: response to operation, Br. Med. J., 1988, vol. 296, pp. 338–339.
Gourtsoyiannis, N., Prassopoulos, P., Cavouras, D., et al., The thickness of the renal parenchyma decreases with age: a CT study of 360 patients, Am. J. Roentgenol., 1990, vol. 155, pp. 541–544.
Greider, C.W. and Blackburn, E.H., Identification of a specific telomere terminal transferase activity in Tetrahymena extracts, Cell, 1985, vol. 43, pp. 405–413.
Harley, C.B., Vaziri, H., Counter, C.M., and Allsopp, R.C., The telomere hypothesis of cellular aging, Exp. Gerontol., 1992, vol. 27, pp. 375–382.
Himmelfarb J. and McMonagle E. Manifestations of oxidant stress in uremia, Blood Purif., 2001, vol. 19, pp. 200–205.
Shu, H.-S., Tai, Y.-Y., Chang, K.-T., et al., Plasma high-sensitivity C-reactive protein level is associated with impaired estimated glomerular filtration rate in hypertensives, Acta Cardiol. Sin., 2015, vol. 31, pp. 91–97.
Jensen, J.S., Myrup, B., Borch-Johnsen, K., et al., Aspects of haemostatic function in healthy subjects with microalbuminuria: a potential atherosclerotic risk factor, Thromb. Res., 1995, vol. 77, pp. 423–430.
Toblli, J.E., Bevione, P., Di Gennaro, F., et al., Understanding the mechanisms of proteinuria: therapeutic implications, Int. J. Nephrol., 2012. doi 10.1155/2012/546039
Ju, Z. and Rudolph, K.L., Telomeres and telomerase in stem cells during aging and disease, Genome Dyn., 2006, vol. 1, pp. 84–103.
Kidney disease: improving global outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease, Kidney Int. Suppl., 2013, vol. 3, pp. 1–150.
Klein, N.J., Shenna, G.I., Heyderman, R.S., and Levin, M., Alteration in glycosaminoglycan metabolism and surface charge on human umbilical vein endothelial cells induced by cytokines, endotoxin and neutrophils, J. Cell. Sci., 1992, vol. 102, pp. 821–832.
Knöbl, P., Schernthaner, G., Schnack, C., et al., Thrombogenic factors are related to urinary albumin excretion rate in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetic patients, Diabetologia, 1993, vol. 36, pp. 1045–1050.
Lauren, P.W. and Schnellmann, R.G., Telomeres and telomerase in renal health, J. Am. Soc. Nephrol., 2011, vol. 22, pp. 39–41.
Levey, A.S., Coresh, J., Greene, T., et al., Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate, Ann. Int. Med., 2006, vol. 145, pp. 247–254.
Lin, J., Hu, F.B., Rimm, E.B., et al., The association of serum lipids and inflammatory biomarkers with renal function in men with type II diabetes mellitus, Kidney Int., 2006, vol. 69, pp. 336–342.
Lindeman, R.D., Overview: renal physiology and pathophysiology of aging, Am. J. Kidney Dis., 1990, no. 16, pp. 275–282.
Melk, A., Ramassar, V., Helms, L.M., et al., Telomere shortening in kidneys with age, J. Am. Soc. Nephrol., 2000, vol. 11, pp. 444–453.
Menon, V., Wang, X., Greene, T., et al., Relationship between C-reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease, Am. J. Kidney Dis., 2003, vol. 42, pp. 44–52.
Njajou, O.T., Hsueh, W.C., Blackburn, E.H., et al., Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study, J. Gerontol., Ser. A, 2009, vol. 64, no. 8, pp. 860–864.
Rule, A.D., Amer, H., Cornell, L., et al., The association between age and nephrosclerosis on renal biopsy among healthy adults, Ann. Int. Med., 2010, vol. 152, pp. 561–567.
Schmitt, R. and Cantley, L.G., The impact of aging on kidney repair, Am. J. Physiol.: Cell Physiol., 2008, vol. 294, pp. F1265–F1272.
Singh, D., Whooley, M., and Shlipak, M., Association of cystatin C and estimated GFR with inflammatory biomarkers: the heart and soul, Care Med., 2008, vol. 36, no. 1, pp. 81–86.
Thijssen, D.H., Vos, J.B., Verseyden, C., et al., Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training, Aging Cell, 2006, vol. 5, pp. 495–503.
Thum, T., Hoeber, S., Froese, S., et al., Age-dependent impairment of endothelial progenitor cells is corrected by growth hormone-mediated increase of insulin- like growth-factor-1, Circ. Res., 2007, vol. 100, pp. 434–443.
Population Division: World Population Ageing 2013, NewYork: United Nations, 2013.
Annual Data Report, U.S. Renal Data System, Bethesda, MD: Natl. Inst. Diabetes Dig. Kidney Dis., 1996.
van der Harst, P., van der Steege, G., De Boer, R.A., et al., Telomere length of circulating leukocytes is decreased in patients with chronic heart failure, J. Am. Coll. Cardiol., 2007, vol. 49, pp. 1459–1464.
Verdun, R.E. and Karlseder, J., Replication and protection of telomeres, Nature, 2007, vol. 447, pp. 924–931.
Vlassara, H., Torreggiani, M., Post, J.B., et al., Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging, Kidney Int. Suppl., 2009, vol. 76, no. 114, pp. S3–S11.
Von Zglinicki, T., Oxidative stress shortens telomeres, Trends Biochem. Sci., 2002, vol. 27, pp. 339–344.
Westhoff, J.H., Schildhorn, C., Jacobi, C., et al., Telomere shortening reduces regenerative capacity after acute kidney injury, J. Am. Soc. Nephrol., 2010, vol. 21, pp. 327–336.
Wills, L.P. and Schnellmann, R.G., Telomeres and telomerase in renal health, J. Am. Soc. Nephrol., 2011, vol. 22, pp. 39–41.
Wong, L.S.M., van der Harst, P., De Boer, R.A., et al., Renal dysfunction is associated with shorter telomere length in heart failure, Clin. Res. Cardiol., 2009, vol. 98, pp. 629–634.
Zakian, V.A., Life and cancer without telomerase, Cell, 1997, vol. 91, pp. 1–3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.S. Pykhtina, I.D. Strazhesko, O.N. Tkacheva, D.U. Akasheva, E.N. Dudinskaya, V.A. Vygodin, E.V. Plokhova, A.S. Kruglikova, S.A. Boitsov, 2016, published in Uspekhi Gerontologii, 2016, Vol. 29, No. 1, pp. 79–85.
Rights and permissions
About this article
Cite this article
Pykhtina, V.S., Strazhesko, I.D., Tkacheva, O.N. et al. Association of renal function, telomere length, and markers of chronic inflammation in patients without chronic kidney and cardiovascular diseases. Adv Gerontol 6, 217–223 (2016). https://doi.org/10.1134/S2079057016030097
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079057016030097