Abstract
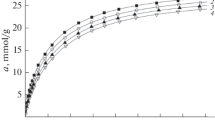
Carbon microporous adsorbents obtained on the basis of polymers are promising adsorbents for the tasks of adsorption storage of natural gas due to the possibility of creating a precise porous structure, as well as optimal mechanical characteristics. A study of the adsorption of methane in a carbon adsorbent based on a composite polymer of furfural and epoxy resin in the temperature range from 178 to 360 K and pressures up to 25 MPa has been carried out. The thermodynamic functions of the adsorption system—the differential molar isosteric and integral heats of adsorption, as well as the isosteric entropy, enthalpy, and heat capacity of the system are calculated. The obtained thermodynamic functions are of fundamental importance in the analysis of the properties of nanodispersed adsorbate in the micropores of the adsorbent, and can also be used as input data in modeling the thermodynamic states of experimental systems for methane storage and transportation.







Similar content being viewed by others
REFERENCES
Tsivadze, A.Yu., Aksyutin, O.E., Ishkov, A.G., et al., Russ. Chem. Rev., 2018, vol. 87, no. 10, pp. 950–983.
Tsivadze, A.Yu., Aksyutin, O.E., Ishkov, A.G., et al., Russ. Chem. Rev., 2019, vol. 88, no. 9, pp. 925–978.
Kumar, K.V., Preuss, K., Titirici, M.M., and Rodríguez-Reinoso, F., Chem. Rev., 2017, vol. 117, no. 3, pp. 1796–1825. https://doi.org/10.1021/acs.chemrev.6b00505
Men’shchikov, I.E., Shiryaev, A.A., Shkolin, A.V., Vysotskii, V.V., Khozina, E.V., and Fomkin, A.A., Korean J. Chem. Eng., 2021, vol. 38, pp. 276–291. https://doi.org/10.1007/s11814-020-0683-2
Men’shchikov, I.E., Fomkin, A.A., Shkolin, A.V., et al., Russ. Chem. Bull., 2018, vol. 67, no. 10, pp. 1814–1822. https://doi.org/10.1007/s11172-018-2294-1
Knyazeva, M.K., Solovtsova, O.V., Tsivadze, A.Yu., et al., Russ. J. Inorg. Chem., 2019, vol. 64, pp. 1507–1512. https://doi.org/10.1134/S0036023619120064
Knyazeva, M.K., Tsivadze, A.Yu., Solovtsova, O.V., et al., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, pp. 9–14. https://doi.org/10.1134/S2070205119010064
Makal, T.A., Li, J.-R., Lu, W., and Zhou, H.-C., Chem. Soc. Rev., 2012, vol. 41, pp. 7761–7779.
Solovtsova, O.V., Shkolin, A.V., Men’shchikov, I.E., et al., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, no. 6, pp. 1080–1084. https://doi.org/10.1134/S2070205119060303
Solovtsova, O.V., Shkolin, A.V., Men’shchikov, I.E., et al., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, no. 5, pp. 826–832. https://doi.org/10.1134/S207020511905023X
Rubio-Martinez, M., Avci-Camur, C., Thornton, A.W., Imaz, I., Maspoch, D., and Hill, M.R., Chem. Soc. Rev., 2017, vol. 46, pp. 3453–3480.
Valizadeh, B., Nguyen, T.N., and Stylianou, K.C., Polyhedron, 2018, vol. 145, pp. 1–15.
Fomkin, A.A., Pribylov, A.A., Tkachev, A.G., et al., Prot. Met. Phys. Chem. Surf., 2020, vol. 56, no. 1, pp. 3–7.
Men’shchikov, I.E., Fomkin, A.A., Tsivadze, A.Y., et al., Adsorption, 2017, vol. 23, no. 2–3, pp. 327–339.
Gur’yanov, V.V., Mukhin, V.M., and Kurilkin, A.A., Catal. Ind., 2013, vol. 5, pp. 156–163.
Mukhin, V.M., Tarasov, A.V., and Klushin, V.N., Aktivnye ugli Rossii (Active Carbons of Russia), Moscow: Metallurgiya, 2000.
Mukhin, V.M., et al., Sorbtsionnye Khromatogr. Protsessy, 2009, vol. 9, no. 2, pp. 191–195.
Casco, M.E., Martínez-Escandell, M., and Gadea-Ramos, E., Chem. Mater., 2015, vol. 27, no. 3, pp. 959–964.
Wang, Y., Ercan, C., Khawajah, A., and Othman, R., AIChE J., 2012, vol. 58, no. 3, pp. 782–788.
Kockrick, E., Schrage, C., Borchardt, L., Klein, N., Rose, M., Senkovska, I., and Kaskel, S., Carbon, 2010, vol. 48, no. 6, pp. 1707–1717.
Men’shchikov, I.E., Shkolin, A.V., Strizhenov, E.M., et al., Nanomaterials, 2020, vol. 10, no. 11, p. 2243. https://doi.org/10.3390/nano10112243
Men’shchikov, I., Shkolin, A., Khozina, E., and Fomkin, A., Nanomaterials, 2020, vol. 10, no. 7, p. 1379. https://doi.org/10.3390/nano10071379
Ridha, F.N., Yunus, R.M., Rashid, M., and Ismail, A.F., Exp. Therm. Fluid Sci., 2007, vol. 32, pp. 14–22.
Feroldi, M., Neves, A.C., Borba, C.E., and Alves, H.J., J. Cleaner Prod., 2018, vol. 172, pp. 921–926.
Sychev, V.V., Vasserman, A.A., Zagoruchenko, V.A., et al., Termodinamicheskie svoistva metana (Thermodynamic Properties of Methane), Moscow: Izd. Standartov, 1979.
Dubinin, M.M., Prog. Surf. Membr. Sci., 1975, vol. 9, pp. 1–70.
Brunauer, S., Emmett, P.H., and Teller, E., J. Am. Chem. Soc., 1938, vol. 60, no. 2, pp. 309–319.
GOST (State Standard) no. R 55959-2014: Activated Carbon. Standard Test Method for Bulk Density, Moscow: Standartinform, 2014.
Shkolin, A.V. and Fomkin, A.A., Russ. Chem. Bull., 2008, vol. 57, pp. 1799–1805.
Pribylov, A.A., Serpinskii, V.V., and Kalashnikov, S.M., Zeolites, 1991, vol. 11, pp. 846–849.
Fomkin, A.A., Shkolin, A.V., Men’shchikov, I.E., et al., Meas. Tech., 2016, vol. 58, no. 12, pp. 1387–1391. https://doi.org/10.1007/s11018-016-0904-6
Bakaev, V.A., Dokl. Akad. Nauk SSSR, 1966, vol. 167, pp. 369–372.
Hill, T.L., in Advances in Catalysis and Related Subjects, Frankerburg, Y.I., , Eds., New York: Academic Press, 1952, vol. 4, pp. 211–258.
Fomkin, A.A., Adsorption, 2005, vol. 11, no. 3, pp. 425–436.
Shkolin, A.V., Fomkin, A.A., and Potapov, S.V., Russ. Chem. Bull., 2017, vol. 66, no. 4, pp. 607–613.
Bakaev, V.A., Doctoral Sci. (Phys.-Math.) Dissertation, Moscow: Moscow State Univ., 1989.
Fomkin, A.A., Men’shchikov, I.E., Pribylov, A.A., et al., Colloid J., 2017, vol. 79, no. 1, pp. 144–151. https://doi.org/10.1134/S1061933X16060053
Fomkin, A.A., Doctoral Sci. (Phys.-Math.) Dissertation, Moscow, 1993.
Anuchin, K.M., Fomkin, A.A., Korotych, A.P., and Tolmachev, A.M., Prot. Met. Phys. Chem. Surf., 2014, vol. 50, no. 2, pp. 173–177.
Shkolin, A.V., Fomkin, A.A., Tsivadze, A.Yu., et al., Prot. Met. Phys. Chem. Surf., 2016, vol. 52, no. 6, pp. 955–963.
Tovbin, Yu.K., in Adsorbtsiya, adsorbenty i adsorbtsionnye protsessy v nanoporistykh materialakh (Adsorption, Adsorbents, and Adsorptive Processes in Nano-Porous Materials), Moscow: Granitsa, 2011.
Fomkin, A.A., Serpinskii, V.V., and Fidler, K., Izv. Akad. Nauk SSSR, Ser. Khim.,1982, no. 6, p. 1207.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Men’shchikov, I.E., Fomkin, A.A. & Shkolin, A.V. Thermodynamics of Methane Adsorption in a Microporous Carbon Adsorbent Prepared From Polymer Composition. Prot Met Phys Chem Surf 57, 883–889 (2021). https://doi.org/10.1134/S2070205121050191
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205121050191