Abstract
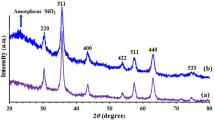
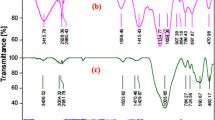
This study addressed the preparation and characterization of polyethylene glycol-substituted 1-methylimidazolium hydroxide supported on magnetic nanoparticles (MNP@PEG-ImOH) by FESEM, FT-IR, EDAX, TEM, TGA, VSM, and XRD techniques. The catalytic activity of MNP@PEG-ImOH has been examined in Knoevenagel condensation between active methylene compounds and aromatic aldehydes in aqueous medium at room temperature. Numerous benefits of the catalytic system, such as higher yields of the products, shorter reaction time, reusability and recyclability of the catalyst, simplified work-up, and more acceptable reaction conditions, have been demonstrated. It is possible to easily isolate the catalyst from the reaction mixture by an external magnet and reapply it in the consequent reactions with no remarkable loss of activity.











Similar content being viewed by others
REFERENCES
Campisciano, V., Giacalone, F., and Gruttadauria, M., Chem. Rec., 2017, vol. 17, p. 918. https://doi.org/10.1002/tcr.201700005
Rodríguez-Pérez, L., Teuma, E., Falqui, A., Gómez, M., and Serp, P., Chem. Commun., 2008, p. 4201. https://doi.org/10.1039/B804969F
Tan, J., Liu, X., Yao, N., Hu, Y.L., and Li, X.H., ChemistrySelect, 2019, vol. 4, p. 2475. https://doi.org/10.1002/slct.201803739
Polesso, B.B., Bernard, F.L., Ferrari, H.Z., Duarte, E.A., Vecchia, F.D., and Einloft, S., Heliyon, 2019, vol. 5, article ID e02183. https://doi.org/10.1016/j.heliyon.2019.e02183
Rostamizadeh, S., Zekri, N., and Tahershamsi, L., Chem. Heterocycl. Compd., 2015, vol. 51, p. 526. https://doi.org/10.1007/s10593-015-1728-z
Wang, T., Wang, W., Lyu, Y., Chen, X., Li, C., Zhang, Y., Song, X., and Ding, Y., RSC Adv., 2017, vol. 7, p. 2836. https://doi.org/10.1039/C6RA26780G
Patel, N., Katheriya, D., Dadhania, H., and Dadhania, A., Res. Chem. Intermed., 2019, vol. 45, p. 5595. https://doi.org/10.1007/s11164-019-03922-0
Ionic Liquids: Applications and Perspectives, Kokorin, A., Ed., Rijeka, Croatia: InTech, 2011.
Hu, Y.L. and Fang, D., J. Mex. Chem. Soc., 2017, vol. 60, p. 207. https://doi.org/10.29356/jmcs.v60i4.113
Tamami, B., Sardarian, A., and Ataollahi, E., Turk. J. Chem., 2016, vol. 40, p. 422. https://doi.org/10.3906/kim-1504-40
Khanapure, S., Jagadale, M., Kale, D., Gajare, S., and Rashinkar, G., Aust. J. Chem., 2019, vol. 72, p. 513. https://doi.org/10.1071/CH18576
Polshettiwar, V., Luque, R., Fihri, A., Zhu, H., Bouhrara, M., and Basset, J.-M., Chem. Rev., 2011, vol. 111, p. 3036. https://doi.org/10.1021/cr100230z
Jiang, Y., Guo, C., **a, H., Mahmood, I., Liu, C., and Liu, H., J. Mol. Catal. B: Enzym., 2009, vol. 58, p. 103. https://doi.org/10.1016/j.molcatb.2008.12.001
Bagheri, M., Masteri-Farahani, M., and Ghorbani, M., J. Magn. Magn. Mater., 2013, vol. 327, p. 58. https://doi.org/10.1016/j.jmmm.2012.09.038
Garkoti, C., Shabir, J., and Mozumdar, S., New J. Chem., 2017, vol. 41, p. 9291. https://doi.org/10.1039/C6NJ03985E
Makosza, M., Pure Appl. Chem., 2000, vol. 72, p. 1399. https://doi.org/10.1351/pac200072071399
Davarpanah, J. and Kiasat, A.R., Catal. Commun., 2013, vol. 42, p. 98. https://doi.org/10.1016/j.catcom.2013.07.040
Ayashi, N., Fallah-Mehrjardi, M., and Kiasat, A.R., Russ. J. Org. Chem., 2017, vol. 53, p. 846. https://doi.org/10.1134/S1070428017060069
Jain, Y., Kumari, M., Agarwal, M., and Gupta, R., Carbohydr. Res., 2019, vol. 482, article ID 107736. https://doi.org/10.1016/j.carres.2019.06.015
Rezvani, M.A., Oghoulbeyk, Z.N., Khandan, S., and Mazzei, H.G., Polyhedron, 2020, vol. 177, article ID 114291. https://doi.org/10.1016/j.poly.2019.114291
Mase, N. and Horibe, T., Org. Lett., 2013, vol. 15, p. 1854. https://doi.org/10.1021/ol400462d
Poor Heravi, M.R. and Piri, S., J. Chem., 2013, vol. 2013, article ID 652805. https://doi.org/10.1155/2013/652805
Ogiwara, Y., Takahashi, K., Kitazawa, T., and Sakai, N., J. Org. Chem., 2015, vol. 80, p. 3101. https://doi.org/10.1021/acs.joc.5b00011
van Schijndel, J., Canalle, L.A., Molendijk, D., and Meuldijk, J., Green Chem. Lett. Rev., 2017, vol. 10, p. 404. https://doi.org/10.1080/17518253.2017.1391881
Kakesh, N., Sayyahi, S., and Badri, R., C. R. Chim., 2018, vol. 21, p. 1023. https://doi.org/10.1016/j.crci.2018.09.009
Shaterian, H.R., Arman, M., and Rigi, F., J. Mol. Liq., 2011, vol. 158, p. 145. https://doi.org/10.1016/j.molliq.2010.11.010
Mochalov, S.S., Chasanov, M.I., Fedotov, A.N., and Zefirov, N.S., Chem. Heterocycl. Compd., 2011, vol. 47, p. 1105. https://doi.org/10.1007/s10593-011-0881-2
Kühbeck, D., Saidulu, G., Reddy, K.R., and Díaz, D.D., Green Chem., 2012, vol. 14, p. 378. https://doi.org/10.1039/C1GC15925A
Levchenko, K.S., Chudov, K.A., Zinoviev, E.V., Lyssenko, K.A., Fakhrutdinov, A.N., Demin, D.U., Poroshin, N.O., Shmelin, P.S., and Grebennikov, E.P., Tetrahedron Lett., 2019, vol. 60, p. 1505. https://doi.org/10.1016/j.tetlet.2019.04.050
Tarade, K., Shinde, S., Sakate, S., and Rode, C., Catal. Commun., 2019, vol. 124, p. 81. https://doi.org/10.1016/j.catcom.2019.03.005
Kiasat, A.R. and Davarpanah, J., J. Mol. Catal. A: Chem., 2013, vol. 373, p. 46. https://doi.org/10.1016/j.molcata.2013.03.003
Amini, A., Sayyahi, S., Saghanezhad, S.J., and Taheri, N., Catal. Commun., 2016, vol. 78, p. 11. https://doi.org/10.1016/j.catcom.2016.01.036
Kassaee, M.Z., Masrouri, H., and Movahedi, F., Appl. Catal., A, 2011, vol. 395, p. 28. https://doi.org/10.1016/j.apcata.2011.01.018
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Fallah-Mehrjardi, M., Behjatmanesh-Ardakani, R. & Saidian, S. Magnetic Nanoparticle-Supported Basic Ionic Liquid: A Reusable Phase-Transfer Catalyst for Knoevenagel Condensation in Aqueous Medium. Russ J Org Chem 58, 144–151 (2022). https://doi.org/10.1134/S1070428022010201
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022010201