Abstract
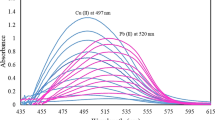
A new and validated method was described for the resolution of a ternary mixture of copper β‑resorcylate (CuR2), lead β-resorcylate (PbR2), and lead oxide in double base (DB) solid propellants without prior separation steps in accordance with military guidelines. Two sets of reaction and determination conditions were developed. The first set was based on reaction of Cu(II) and Pb(II) with 4-(2-pyridylazo)resorcinol reagent in alkaline media and using derivative ratio spectrophotometry for the simultaneous determination of Cu(II) and Pb(II) by measuring the peak intensities at 539 and 543 nm, respectively. In the second set, with hydrolysis of CuR2 and PbR2 in acetic acid medium, the released resorcylic acid was determined via complex formation of resorcylic acid with Fe(III) and absorbance measurement at 526 nm. The amounts of CuR2 and total lead can be determined by derivative ratio spectrophotometry. So, according to the obtained data for resorcylic acid, CuR2, and total lead by derivative ratio spectrophotometry, the amounts of PbR2 and PbO were determined by applying stoichiometric equations. The proposed method was successfully applied for the determination of CuR2, PbR2, and PbO in DB propellants. The results of the method were statistically compared based on t- and F-tests with those obtained by inductively coupled plasma atomic emission spectrometry. The results showed that the proposed method offers an accurate and reliable approach for the determination of these compounds in DB propellants and can be suggested as a routine method in military quality control laboratories.







Similar content being viewed by others
REFERENCES
Saraji, M. and Boroujeni, M.K., Anal. Bioanal. Chem., 2014, vol. 406, no. 8, p. 2027.
Al-Saidi, H. and Emara, A.A., J. Saudi Chem. Soc., 2014, vol. 18, no. 6, p. 745.
Aly, A.A. and Gorecki, T., Molecules, 2020, vol. 25, no. 7, p. 1719.
Pena-Pereira, F., Lavilla, I., and Bendicho, C., Spectrochim. Acta, Part B, 2009, vol. 64, no. 1, p. 1.
Awad, T., Moharram, H., Shaltout,O., Asker, D., and Youssef, M., Food Res. Int., 2012, vol. 48, no. 2, p. 410.
Majid, I., Nayik,G. A., and Nanda, V., Cogent Food Agric., 2015, vol. 1, no. 1, p. 0.
EI-Yazbi, A.F., Elashkar, N.E., Abdel-Hay, K.M., Ahmed, H.M., and Talaat, W., Anal. Sci. Technol., 2021, vol. 12, no. 7, p. 1.
Gałuszka, A., Migaszewski, Z., and Namiesnik, J., TrAC, Trends Anal. Chem., 2013. vol. 50, p. 78.
Hajian, R., Shams, N., and Kaedi, I., J. Chem., 2010, vol. 7, no. 4, p. 1530.
**ao, N., Deng, J., Huang, K., Ju, S., Hu, C., and Liang, J., Spectrochim. Acta, Part A, 2014, vol. 128, p. 312.
Erk, N., Spectrosc. Lett., 2001, vol. 34, no. 6, p. 745.
Abdel-Hay, M.H., Gazy, A.A., Hassan, E.M., and Belal. T.S., J. Chin. Chem. Soc., 2008, vol. 55, no. 5, p. 971.
Agrawal, J.P., High Energy Materials: Propellants, Explosives and Pyrotechnics, Berlin: Wiley, 2010.
Varghese, T. and Krishnamurthy, V., The Chemistry and Technology of Solid Rocket Propellants (A Treatise on Solid Propellants), Delhi: Allied, 2017.
Camp, A.T. and Csanady, E.R., Doublebase ballistic modifiers, US Patent 4420350, 1980.
Berteleau, G., Fonblanc, G., Longevialle, Y., and Rat, M., Compositions modifying ballistic properties and propellants containing such compositions, US Patent 5639987, 1997.
Neidert, J. B. and Williams. M., Castable double base solid rocket propellant containing ballistic modifier pasted in an inert polymer, US Patent 6024810, 1998.
Joshi, A. and Singh, H., J. Energ. Mater., 1992, vol. 10, nos. 4–5, p. 299.
Yu, L. and You-zhi, L., Ind. Catal., 2007, vol. 15, no. 6, p. 66.
Hao, G., Liu, J., **ao, L., Gao, H. Qiao,Y., Jiang,W., Zhao, F., and Gao, H., J. Therm. Anal. Calorim., 2016, vol. 124, no. 3, p. 1367.
Warren, L.R., Pulham,C.R., and Morrison, C.A., Phys. Chem. Chem. Phys., 2020, vol. 22, no. 44, p. 25502.
Warren, L.R., Wang, Z., Pulham,C.R., and Morrison, C.A., Propellants, Explos., Pyrotech., 2021, vol. 46, no. 1, p. 13.
Hewkin, D.J., Hicks, J., Powling, J., and Watts, H., Combust. Sci. Technol., 1971, vol. 2, nos. 5–6, p. 307.
de Souza, R.M., Leocadio, L.G., and da Silveira, C.L.P., Anal. Lett., 2008, vol. 4, no. 9, p. 1615.
Wuilloud, R.G., Acevedo, H. Vazquez, F., and Martinez, L.D., Anal. Lett., 2002, vol. 35, no. 10, p. 1649.
Grinshtein, I.L., Vasileva, L.A., and Maksimova, Yu.V., J. Anal. Chem., 2003, vol. 58, no. 7, p. 622.
Bagherian, G., Chamjangali, M.A., Evari, H.S., and Ashrafi, M., Anal. Sci. Technol., 2019, vol. 10, no. 1, p.1.
Khuhawar, M., Yazdi, A.S., and Uden, P., Chromatographia, 2002, vol. 56, p. 11.
Pereiro, R.I. and Díaz, C.A., Anal. Bioanal. Chem., 2002, vol. 372, no. 1, p. 74.
Meng, S., **g, B., Fan, Y., Liu, Y., and Guo, Y., J. Anal. Chem., 2009, vol. 64, no. 11, p. 1108.
Malik, A.K. and Rao, A.L.J., J. Anal. Chem., 2000, vol. 55, no. 8, p. 746.
MIL-STD-286C w/CHANGE (Militry Standard): Propellants, Solid: Sampling, Examination and Testing, 2010.
Frys, O., Cesla, P., Bajerova, P., Adam, M., and Ventura, K., Talanta, 2012, vol. 99, p. 316.
Ghasemi, J., Peyman, H., and Meloun, M., J. Chem. Amp. Eng. Data, 2007, vol. 52, no. 4, p. 1171.
Shivahare, G., Mathur, S., and Mathur, M., Anal. Bioanal. Chem., 1972, vol. 261, no. 2, p. 126.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Zarei, A., Mardi, K. Development of Derivative Ratio Spectrophotometric Method for Simultaneous Determination of Copper β-resorcylate, Lead β-resorcylate, and Lead Oxide in Double base Propellants. J Anal Chem 77, 1247–1255 (2022). https://doi.org/10.1134/S1061934822100161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934822100161