Abstract
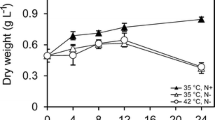
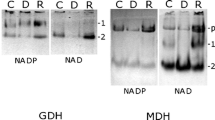
The effect of nitrogen starvation and, for the first time, low temperature, as well as their simultaneous effect, on the physiology and ultrastructure of cells of microalgae of the genus Lobosphaera (Chlorophyta, Trebouxiophyceae) was studied. Nitrogen deficiency in both strains led to a decrease in the content of chlorophyll by three times and an increase in the proportion of carotenoids by two times. A decrease in the content of both chlorophyll and carotenoids was observed at +10°C. The simultaneous effect of two factors resulted in a threefold decrease in the chlorophyll content in NAMSU 924/2 and a sixfold decrease in NAMSU (CALU) 1497; the proportion of carotenoids in both strains decreased by 1.5–2 times. Data on ultrastructural changes in cells of microalgae of the genus Lobosphaera under the influence of stress factors have been obtained. A similar nature of the response in both strains to stress conditions was noted. Nitrogen deficiency led to the accumulation of numerous lipid droplets in the cytoplasm of cells along the cell wall. Long-term incubation on a nitrogen-free medium led to the filling of the entire volume of cells with lipid droplets, disassembly of the membrane system of chloroplasts, that reduction in sizeand being located between densely lying lipid droplets. At low temperatures, the number of thylakoids decreased, while the interthylakoid space and the size of chloroplasts increased. With simultaneous exposure to nitrogen starvation and low temperature, numerous lipid droplets accumulated, the number of thylakoids decreased, the interthylakoid space and the size of the chloroplast increased, which was noted under separate exposure to stress factors. The pyrenoid in both strains did not undergo significant changes in all cases.





Similar content being viewed by others
REFERENCES
Borowitzka, M.A., Microalgae for aquaculture: opportunities and constraints, J. Appl. Phycol., 1997, vol. 9, p. 393. https://doi.org/10.1023/A:1007921728300
Michalak, I. and Chojnacka, K., Algal extracts: technology and advances, Eng. Life Sci., 2014, vol. 14, p. 581. https://doi.org/10.1002/elsc.201400139
Chojnacka, K., Wieczorek, P., Schroeder, G., and Michalak, I., Algae Biomass: Characteristics and Applications. Towards Algae-Based Products, Springer, 2018, vol. 8. https://doi.org/10.1007/978-3-319-74703-3
Solovchenko, A.E., Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses, Russ. J. Plant Physiol., 2012, vol. 59, p. 167.
Guschina, I.A. and Harwood, J.L., Lipids and lipid metabolism in eukaryotic algae, Prog. Lipid Res., 2006, vol. 45, p. 160. https://doi.org/10.1016/j.plipres.2006.01.001
Morgan-Kiss, R.M., Priscu, J., Pocock, T., Gudynaite-Savitch, L., and Norman, P.A., Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments, Microbiol. Mol. Biol. Rev., 2006, vol. 70, p. 222. https://doi.org/10.1128/MMBR.70.1.222-252.2006
Cohen, Z., Khozin-Goldberg, I., Adlerstein, D., and Bigogno, C., The role of triacylglycerol as a reservoir of polyunsaturated fatty acids for the rapid production of chloroplastic lipids in certain microalgae, Biochem. Soc. Trans., 2000, vol. 28, p. 740. https://doi.org/10.1042/bst0280740
Spolaore, P., Joannis-Cassan, C., Duran, E., and Isambert, A., Commercial applications of microalgae, J. Biosci. Bioeng., 2006, vol. 101, p. 87. https://doi.org/10.1042/bst0280740
Ibañez, E. and Cifuentes, A., Benefits of using algae as natural sources of functional ingredients, J. Sci. Food Agric., 2013, vol. 93, p. 703. https://doi.org/10.1002/jsfa.6023
Ścieszka, S. and Klewicka, E., Algae in food: A general review, Crit. Rev. Food Sci. Nutr., 2019, vol. 59, p. 3538. https://doi.org/10.1080/10408398.2018.1496319
Mudimu, O., Koopmann, I.K., Rybalka, N., Friedl, T., Schulz, R., and Bilger, W., Screening of microalgae and cyanobacteria strains for α-tocopherol content at different growth phases and the influence of nitrate reduction on α-tocopherol production, J. Appl. Phycol., 2017, vol. 29, p. 2867. https://doi.org/10.1007/s10811-017-1188-1
Mal’tsev, E.I., Mal’tseva, I.A., Mal’tseva, S.Yu., and Kulikovskiy, M.S., Biotechnological potential of a new strain of Bracteacoscus bulatus (Sphaeropleales, Chlorophyta) as a promising producer of omega-6 polyunsaturated fatty acids, Fiziol. Rast., 2020, vol. 67, p. 96. https://doi.org/10.31857/S0015330320010121
Stanier, R.Y., Kunisawa, R., Mandel, M., and Cohen-Bazire, G., Purification and properties of unicellular blue-green algae (order Chroococcales), Bacteriolog. Rev., 1971, vol. 35, p. 171. https://doi.org/10.1128/br.35.2.171-205.1971
Gorelova, O.A., Baulina, O.I., Solovchenko, A.E., Chekanov, K.A., Chivkunova, O.B., Fedorenko, T.A., and Lobakova, E.S., Similarity and diversity of the Desmodesmus spp. microalgae isolated from associations with White Sea invertebrates, Protoplasma, 2015, vol. 252, p. 489. https://doi.org/10.1007/s00709-014-0694-0
Reynolds, E.S., The use of lead citrate of high pH as an electron opaque strain in electron microscopy, J. Cell. Biol., 1963, vol. 17, p. 208. https://doi.org/10.1083/jcb.17.1.208
Folch, J., Lees, M., and Stanley, G.S., A simple method for the isolation and purification of total lipids from animal tissues, J. Biol. Chem., 1957, vol. 226, p. 497. https://doi.org/10.1016/S0021-9258(18)64849-5
Kates, M., Lipid extraction procedures, in Techniques of Lipidology: Isolation, Analysis and Identification of Lipids, Burden, R.H. and van Knippenberg, P.H., Eds., Amsterdam: Elsevier Science, 1986, 2nd ed., p. 106.
Wellburn, A.R., The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution, J. Plant Physiol., 1994, vol. 144, p. 307. https://doi.org/10.1016/S0176-1617(11)81192-2
Steward, F.C. and Mühlethaler, K., The structure and development of the cell-wall in the Valoniaceae as revealed by the electron microscope, Ann. Bot., 1953, vol. 17, p. 295. https://doi.org/10.1093/oxfordjournals.aob.a083351
Lichtenthaler, H.K., Chlorophylls and carotenoids: pigments of photosynthetic biomembranes, Meth. Enzym., 1987, vol. 148, p. 350. https://doi.org/10.1016/0076-6879(87)48036-1
Cunningham, Jr.F.X. and Gantt, E., Genes and enzymes of carotenoid biosynthesis in plants, Annu. Rev. Plant Biol., 1998, vol. 49, p. 557. https://doi.org/10.1146/annurev.arplant.49.1.557
Takaichi, S., Carotenoids in algae: distributions, biosynthesis and functions, Marine drugs, 2011, vol. 9, p. 1101. https://doi.org/10.3390/md9061101
Borowitzka, M., Algal biotechnology products and processes—matching science and economics, J. Appl. Phycol., 1992, vol. 4, p. 267. https://doi.org/10.1007/BF02161212
Bigogno, C., Khozin-Goldberg, I., Boussiba, S., Vonshak, A., and Cohen, Z., Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid, Phytochem., 2002, vol. 60, p. 497. https://doi.org/10.1016/S0031-9422(02)00100-0
Leman, J., Oleaginous microorganisms: an assessment of the potential, Adv. Appl. Microbiol., 1997, vol. 43, p. 195. https://doi.org/10.1016/S0065-2164(08)70226-0
Goodson, C.R.R., Wang, Z.T., and Goodenough, U., Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost, Eukaryotic cell, 2011, vol. 10, p. 1592. https://doi.org/10.1128/EC.05242-11
Fan, J., Andre, C., and Xu, C., A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii, FEBS Letters, 2011, vol. 585, p. 1985. https://doi.org/10.1016/j.febslet.2011.05.018
Schüler, L.M., Schulze, P.S., Pereira, H., Barreira, L., León, R., and Varela, J., Trends and strategies to enhance triacylglycerols and high-value compounds in microalgae, Algal Res., 2017, vol. 25, p. 263. https://doi.org/10.1016/j.algal.2017.05.025
Kugler, A., Zorin, B., Didi-Cohen, S., Sibiryak, M., Gorelova, O., Ismagulova, T., Kokabi, K., Kumari, P., Lukyanov, A., Boussiba, S., Solovchenko, A., and Khozin-Goldberg, I., Long-chain polyunsaturated fatty acids in the green microalga Lobosphaera incisa contribute to tolerance to abiotic stresses, Plant Cell Physiol., 2019, vol. 60, p. 1205. https://doi.org/10.1093/pcp/pcz013
Merzlyak, M.N., Chivkunova, O.B., Gorelova, O.A., Reshetnikova, I.V., Solovchenko, A.E., Khozin-Goldberg, I., and Cohen, Z., Effect of nitrogen starvation on optical properties, pigments, and arachidonic acid content of the unicellular green alga Parietochloris incisa (Trebouxiophyceae, Chlorophyta), J. Phycol., 2007, vol. 43, p. 833. https://doi.org/10.1111/j.1529-8817.2007.00375.x
Solovchenko, A.E., Khozina-Goldberg, I., Didi-Cohen, S., Cohen, Z., and Merzlyak, M.N., Effects of light and nitrogen starvation on the content and composition of carotenoids of the green microalga Parietochloris incisa, Russ. J. Plant Phys., 2008, vol. 55, p. 455.
Solovchenko, A.E., Merzlyak, M.N., Chivkunova, O.B., Reshetnikova, I.V., Khozina-Goldberg, I., Didi-Cohen, S., and Cohen, Z., Effects of illumination and nitrogen starvation on accumulation of arachidonic acid by the microalga Parietochloris incisa, Mosc. Univ. Biol. Sci. Bull., 2008b, vol. 63, p. 44.
Temraleeva, A.D., Green algae species Bracteacoscus bulatus and B. occidentalis (Sphaeropleales, Chlorophyta) new to the soil algae flora of Russia, Vopr. Sovrem. Al’gol., 2018, vol. 1, p. 14.
Watanabe, S., Hirabayashi, S., Boussiba, S., Cohen, Z., Vonshak, A., and Richmond, A. Parietochloris incisa comb. nov. (Trebouxiophyceae, Chlorophyta), Phycolog. Res., 1996, vol. 44, p. 107. https://doi.org/10.1111/j.1440-1835.1996.tb00383.x
Khozin-Goldberg, I., Shrestha, P., and Cohen, Z., Mobilization of arachidonyl moieties from triacylglycerols into chloroplastic lipids following recovery from nitrogen starvation of the microalga Parietochloris incisa, BBA—Mol. Cell Biol. Lipids, 2005, vol. 1738, p. 63. https://doi.org/10.1016/j.bbalip.2005.09.005
Funding
This work was financially supported by the Russian Science Foundation, grant no. 20–74–10028 and performed within the framework of the Interdisciplinary Scientific and Educational School of Moscow University Molecular Technologies of Living Systems and Synthetic Biology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving humans and animals as research subjects.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by M. Shulskaya
Rights and permissions
About this article
Cite this article
Shibzukhova, K.A., Chivkunova, O.B. & Lobakova, E.S. The effect of Low Temperature and Nitrogen Starvation on the Morphological and Physiological Characteristics of Two Strains of Green Microalgae of the Genus Lobosphaera sp. (Chlorophyta, Trebouxiophyceae). Russ J Plant Physiol 70, 55 (2023). https://doi.org/10.1134/S1021443722700091
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722700091