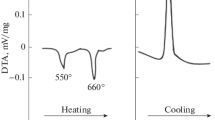
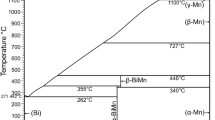
Abstract
Using the methods of differential thermal analysis and X-ray powder diffraction, the Т–х phase diagram of the Li2O⋅2B2O3–Yb2O3⋅B2O3 system has been constructed. Phase diagram of this system belongs to the eutectic type and is characterized by the formation of limited Li2O⋅2B2O3–based solid solutions. The region of limited (1 – х)Li2O⋅B2O3–хYb2O3⋅B2O3 solid solutions at 298–1038 K is x = 0–0.1 mole fraction of Yb2O3⋅B2O3 (or α-Li2O⋅2B2O3). Eutectic coordinates: 1038 K and ~17 mol % Yb2O3⋅B2O3. Depending on the thermal history, (1 – х)Li2O⋅2B2O3–хYb2O3⋅B2O3 (x = 0–0.1) solid solutions are formed both in the form of polycrystals (annealed) and in the form of amorphous glasses (quenched). Glassy and polycrystalline samples of solid solutions α-Li2O⋅2B2O3 (x = 0, 0.05, and 0.1) were synthesized and their physico-chemical properties were studied. The temperature dependence of the standard Gibbs energy of the reaction of formation of solid solutions is estimated (0 ≤ х ≤ 0.1 at 573–1073 K). It is shown that the formation reaction of a solid solution based on Li2O⋅2B2O3 in the range 0 ≤ х ≤ 0.1 mole fraction Yb2O3⋅B2O3 is thermodynamically possible under standard conditions. Measured the electrical properties of (1 – х)Li2O⋅2B2O3–хYb2O3⋅B2O3 (x = 0, 0.05, 0.1) glass samples solid solutions (x = 0–0.1) in the temperature range from 298 to 575 K. The temperature dependences of the direct current (dc) conductivity of α-Li2O⋅2B2O3–samples with different thermal histories (annealing or quenching) differ from each other. It was shown that the introduction of Yb2O3⋅B2O3 (x = 0–0.1) into (1 – х)Li2O⋅2B2O3–хYb2O3⋅B2O3 glasses reduces the conductivity of the samples. The activation energies of conductivity of the glass samples are determined (66.6–90.7 kJ/mol). At temperatures >575 K, the conductivity of α-Li2O⋅2B2O3 glasses deviates from the Arrhenius equation.





Similar content being viewed by others
REFERENCES
M. M. Asadov and E. S. Kuli-zade, J. Alloys Compd. 5, 23 (2020). https://doi.org/10.1016/j.jallcom.2020.155632
N. Nitta, F. Wu, J. T. Lee, et al., Mater. Today 18, 252 (2015). https://doi.org/10.1016/j.mattod.2014.10.040
S. V. Pershina, A. A. Raskovalov, B. D. Antonov, et al., Russ. J. Phys. Chem. A 90, 2194 (2016). https://doi.org/10.1134/S0036024416110200
B. Raguenet, G. Tricot, G. Silly, et al., Solid State Ionics 208, 25 (2012). https://doi.org/10.1016/j.ssi.2011.11.034
N. B. Pimentel, V. R. Mastelaro, J.-C. M’Peko, et al., J. Non-Cryst. Solids 499, 272 (2018). https://doi.org/10.1016/j.jnoncrysol.2018.07.024
A. E. Medvedeva, L. S. Pechen, E. V. Makhonina, et al., Russ. J. Inorg. Chem. 64, 829 (2019). https://doi.org/10.1134/S003602361907012X
L. D. Pye, V. D. Fréchette, and N. J. Kreidl, Borate Glasses: Structure, Properties, and Application (Plenum, New York, 1978). https://doi.org/10.1007/978-1-4684-3357-9
F. Berkemeier, M. Shoar Abouzari, and G. Schmitz, Appl. Phys. Lett. 90, 113110 (2007). https://doi.org/10.1063/1.2713138
R. Korthauer, Lithium-Ion Batteries. Basics and Applications (Springer, Germany, 2018). https://doi.org/10.1007/978-3-662-53071-9
P. Knauth, Solid State Ionics 180, 911 (2009). https://doi.org/10.1016/j.ssi.2009.03.022
A. Felts, Amorphous Inorganic Materials and Glasses (VCH, Weinheim, 1983).
A. V. Egorysheva, V. D. Volodin, and T. Milenov, Russ. J. Inorg. Chem. 50, 1810 (2005). https://doi.org/10.1134/S0036023610110185
B. Douglas and S.-H. Ho, Structure and Chemistry of Crystalline Solids (Springer, Berlin, 2006).
M. M. Asadov and N. A. Akhmedova, Russ. J. Inorg. Chem. 63, 1617 (2018). https://doi.org/10.1134/S0036023618120021
M. M. Asadov, A. N. Mammadov, D. B. Tagiev, et al., Mater. Res. Soc. Symp. Proc. 1765, 115 (2015). https://doi.org/10.1557/opl.2015.816
V. T. Maltsev and S. A. Kutolin, Neorg. Khim. 24, 12 (1979).
M. I. Zargarova, N. A. Akhmedova, and E. S. Kuli-zade, Neorg. Khim. 40, 1389 (1995).
G. A. Sycheva and I. G. Polyakova, Glass Phys. Chem. 41, 590 (2015). https://doi.org/10.1134/S1087659615060152
A. C. M. Rodrigues, R. Keding, and C. Russel, J. Non-Cryst. Solids 273, 53 (2000). https://doi.org/10.1016/S0022-3093(00)00143-5
J. C. Christian, A. Mauger, and A. V. K. Zaghib, Lithium Batteries. Science and Technology (Springer Int., Switzerland, 2016). https://doi.org/10.1007/978-3-319-19108-9
Sh. A. Gamidova, Russ. J. Inorg. Chem. 54, 141 (2009). https://doi.org/10.1134/S0036023609010240
C. Chen, Y. Wu, A. Jiang, et al., J. Opt. Soc. Am. B: Opt. Phys. 6, 616 (1989). https://doi.org/10.1364/JOSAB.6.000616
B. S. R. Sastry, and F. A. Hummel, J. Am. Ceram. Soc. 42, 218 (1959). https://doi.org/10.1111/j.1151-2916.1958.tb13496.x
Zh. G. Bazarova, A. I. Nepomnyashchikh, A. A. Kozlov, et al., Russ. J. Inorg. Chem. 52, 1971 (2007). https://doi.org/10.1134/S003602360712025X
M. M. Asadov and N. A. Akhmedova, Int. J. Thermophys. 35, 1749 (2014). https://doi.org/10.1007/s10765-014-1673-6
S. F. Radaev, L. A. Murady, L. F. Malakhova, et al., Sov. Phys. Crystallogr. 34, 842 (1989).
R. S. Bubnova and S. K. Filatov, High-Temperature Crystal Chemistry of Borates and Borosilicates (Nauka, St. Petersburg, 2008) [in Russian].
G. W. H. Hohne, W. F. Hemminger, and H. J. Flammersheim, Differential Scanning Calorimetry, 2nd ed. (Springer, Berlin, 2003)
J. R. Macdonald and W. B. Johnson, in Impedance Spectroscopy Theory, Experiment, and Applications, Ed. by E. Barsoukov and J. R. Macdonald, 2nd ed. (Wiley, New Yorkk, 2005), p. 1.
G. F. Voronin and I. A. Zaitseva, Russ. J. Phys. Chem. 70, 1116 (1996).
G. F. Voronin, Inorg. Mater. 36, 271 (2000).
I. A. Uspenskaya, V. I. Goryacheva, and I. B. Kutsenok, Russ. J. Phys. Chem. 75, S135 (2001).
I. A. Uspenskaya, V. I. Goryacheva, R. V. Shersten, et al., Russ. J. Phys. Chem. 76, 1581 (2002).
Thermal Constants of Substances, The Handbook, Ed. V. P. Glushko (VINITI AN USSR, Moscow, 1978), No. 8, Part 1 [in Russian].
V. P. Glushko, Thermal Constants of Substances, Database. http://www.chem.msu.su/cgi-bin/tkv.pl?show=welcome.html.
O. Kubaschewski and C. B. Alcook, Metallurgical Thermochemistry, 5th ed. (Pergamon, Oxford, 1979).
I. Barin and G. Platzki, Thermochemical Data of Pure Substances, 3rd ed. (VCH, Weinheim, 1995).
A. G. Morachevskiy and Ye. G. Firsova, Physical Chemistry. Thermodynamics of Chemical Reactions, The Manual (Lan, St. Petersburg, 2015) [in Russian].
S. Murugavel, Phys. B (Amsterdam, Neth.) 72, 134204 (2005). https://doi.org/10.1103/PhysRevB.72.134204
A. N. Cormack, J. Du, and T. R. Zeitler, Phys. Chem. Chem. Phys. 4, 3193 (2002). https://doi.org/10.1039/b201721k
ACKNOWLEDGMENTS
This work was supported by the Science Development Fund under the President of the Republic of Azerbaijan (grant nos. EİF-BGM-3-BRFTF-2+/2017-15/05/1-M-13 and EİF-BGM-4-RFTF-1/2017-21/05/1-M-07).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Asadov, M.M., Mustafaeva, S.N. & Ahmedova, N.A. Phase Equilibria in the Quasibinary Li2O⋅2B2O3–Yb2O3⋅B2O3 System, Assessment of Stability, and Conductivity of Solid Solutions Based on Li2O⋅2B2O3. Russ. J. Phys. Chem. 96, 508–514 (2022). https://doi.org/10.1134/S0036024422030050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422030050