Abstract
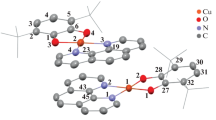
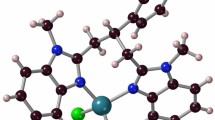
Copper(II) complexes based on 3,5-di-tert-octyl-o-benzoquinone (3,5-t-Oc-Q) have been synthesized. Derivatives of the composition (3,5-t-Oc-SQ)2Cu (I), (3,5-t-Oc-Cat)Cu(Phen) (II), (3,5‑t-Oc-Cat)Cu(DPQ) (III), and (3,5-t-Oc-Cat)Cu(DPPZ) (IV) have been obtained and characterized, where 3,5-t-Oc-SQ is the radical anion of 3,5-di-tert-octyl-o-benzoquinone, 3,5-t-Oc-Cat is the 3,5-di-tert-octyl-o-benzoquinone dianion, Phen is phenanthroline, DPQ is dipyrido[3,2-d:2′,3′-f]quinoxaline, and DPPZ is dipyrido[3,2-a:2′,3′-c]phenazine. The molecular and crystal structures of complexes I and II have been determined by X-ray diffraction. The spectral characteristics of the synthesized copper(II) derivatives have been studied using electron absorption spectroscopy. Crystallographic data for compounds I and II have been deposited with the Cambridge Structural Database (nos. 2291614 and 2279045 for I and II, respectively).




Similar content being viewed by others
REFERENCES
A. Nakada, T. Matsumoto, and H.-C. Chang, Coord. Chem. Rev. 473, 214804 (2022). https://doi.org/10.1016/j.ccr.2022.214804
P. Chaudhuri, C. N. Verani, E. Bill, et al., J. Am. Chem. Soc. 123, 2213 (2001). https://doi.org/10.1021/ja003831d
R. Mukherjee, Inorg. Chem. 59, 12961 (2020). https://doi.org/10.1021/acs.inorgchem.0c00240
S. Sproules and K. Wieghardt, Coord. Chem. Rev. 254, 1358 (2010). https://doi.org/10.1016/j.ccr.2009.12.012
R. Eisenberg, Coord. Chem. Rev. 255, 825 (2011). https://doi.org/10.1016/j.ccr.2010.09.003
R. Eisenberg and H. B. Gray, Inorg. Chem. 50, 9741 (2011). https://doi.org/10.1021/ic2011748
T. Kusamoto and H. Nishihara, Coord. Chem. Rev. 380, 419 (2019). https://doi.org/10.1016/j.ccr.2018.09.012
W. Kaim and B. Schwederski, Coord. Chem. Rev. 254, 1580 (2010). https://doi.org/10.1016/j.ccr.2010.01.009
B. I. Kharisov, M. A. Méndez-Rojas, A. D. Garnovskii, et al., J. Coord. Chem. 55, 745 (2002). https://doi.org/10.1080/0095897022000001511
S. V. Baryshnikova and A. I. Poddel’sky, Molecules 27, 3928 (2022).
N. Monni, M. S. Angotzi, M. Oggianu, et al., J. Mater. Chem. C 10, 1548 (2022). https://doi.org/10.1039/d1tc05335c
C. G. Pierpont, Coord. Chem. Rev. 216–217, 99 (2001). https://doi.org/10.1016/S0010-8545(01)00309-5
S. V. Baryshnikova, A. I. Poddel’sky, E. V. Bellan, et al., Inorg. Chem. 59, 6774 (2020). https://doi.org/10.1021/acs.inorgchem.9b03757
S. V. Baryshnikova, E. V. Bellan, A. I. Poddel’skii, et al., Dokl. Chem. 474, 101 (2017). https://doi.org/10.1134/S0012500817050019
A. V. Piskunov, A. V. Maleeva, A. S. Bogomyakov, et al., Polyhedron 102, 715 (2015). https://doi.org/10.1016/j.poly.2015.10.045
A. V. Piskunov, A. V. Maleeva, G. K. Fukin, et al., Inorg. Chim. Acta 455, 213 (2017). https://doi.org/10.1016/j.ica.2016.10.030
E. V. Bellan, A. I. Poddel’sky, N. A. Protasenko, et al., Inorg. Chem. Commun. 50, 1 (2014). https://doi.org/10.1016/j.inoche.2014.10.001
C. G. Pierpont, Coord. Chem. Rev. 219–221, 415 (2001). https://doi.org/10.1016/S0010-8545(01)00342-3
E. V. Bellan, A. I. Poddel’sky, N. A. Protasenko, et al., ChemistrySelect 1, 2988 (2016). https://doi.org/10.1002/slct.201600506
N. A. Protasenko and A. I. Poddel’sky, Theor. Exp. Chem 56, 338 (2020). https://doi.org/10.1007/s11237-020-09663-1
C. G. Pierpont and R. M. Buchanan, Coord. Chem. Rev. 38, 45 (1981). https://doi.org/10.1016/S0010-8545(00)80499-3
T. Tezgerevska, K. G. Alley, and C. Boskovic, Coord. Chem. Rev. 268, 23 (2014). https://doi.org/10.1016/j.ccr.2014.01.014
A. V. Maleeva, O. Y. Trofimova, I. A. Yakushev, et al., Russ. J. Coord. Chem. 49, 420 (2023). https://doi.org/10.1134/S1070328423600134
I. V. Ershova, A. V. Maleeva, R. R. Aisin, et al., Russ. Chem. Bull. 72, 193 (2023). https://doi.org/10.1007/s11172-023-3724-2
K. I. Pashanova, I. V. Ershova, O. Y. Trofimova, et al., Molecules 27, 8175 (2022). https://doi.org/10.3390/molecules27238175
A. V. Maleeva, I. V. Ershova, O. Y. Trofimova, et al., Mendeleev Commun. 32, 83 (2022). https://doi.org/10.1016/j.mencom.2022.01.027
M. L. Kirk, D. A. Shultz, A. R. Marri, et al., J. Am. Chem. Soc. 144, 21005 (2022). https://doi.org/10.1021/jacs.2c09680
K. I. Pashanova, V. O. Bitkina, I. A. Yakushev, et al., Molecules 26, 4622 (2021). https://doi.org/10.3390/molecules26154622
M. L. Kirk, D. A. Shultz, P. Hewitt, et al., Chem. Sci. 12, 13704 (2021). https://doi.org/10.1039/D1SC02965G
M. L. Kirk, D. A. Shultz, J. Chen, et al., J. Am. Chem. Soc. 143, 10519 (2021). https://doi.org/10.1021/jacs.1c04149
A. V. Cherkasova, K. A. Kozhanov, A. A. Zolotukhin, et al., Russ. J. Coord. Chem. 45, 489 (2019). https://doi.org/10.1134/S1070328419070029
S. Sobottka, M. Nößler, A. L. Ostericher, et al., Chem.—Eur. J. 26, 1314 (2020). https://doi.org/10.1002/chem.201903700
L. Chiang, K. Herasymchuk, F. Thomas, et al., Inorg. Chem. 54, 5970 (2015). https://doi.org/10.1021/acs.inorgchem.5b00783
T. Kurahashi and H. Fujii, J. Am. Chem. Soc. 133, 8307 (2011). https://doi.org/10.1021/ja2016813
C. L. Linfoot, P. Richardson, K. L. McCall, et al., Solar Energy 85, 1195 (2011). https://doi.org/10.1016/j.solener.2011.02.023
Q. Miao, J. Gao, Z. Wang, et al., Inorg. Chim. Acta 376, 619 (2011). https://doi.org/10.1016/j.ica.2011.07.046
K. Neuthe, C. S. Popeney, K. Bialecka, et al., Polyhedron 81, 583 (2014). https://doi.org/10.1016/j.poly.2014.07.015
L. A. Cameron, J. W. Ziller, and A. F. Heyduk, Chem. Sci. 7, 1807 (2016). https://doi.org/10.1039/C5SC02703A
N. Deibel, D. Schweinfurth, J. Fiedler, et al., Dalton Trans. 40, 9925 (2011). https://doi.org/10.1039/C1DT10856E
K. Tahara, Y. Ashihara, T. Higashino, et al., Dalton Trans. 48, 7367 (2019). https://doi.org/10.1039/C8DT05057K
N. F. Romashev, P. A. Abramov, I. V. Bakaev, et al., Inorg. Chem. 61, 2105 (2022). https://doi.org/10.1021/acs.inorgchem.1c03314
N. Deibel, D. Schweinfurth, S. Hohloch, et al., Chem. Commun. 48, 2388 (2012). https://doi.org/10.1039/C2CC15245B
J. Yang, D. K. Kersi, C. P. Richers, et al., Inorg. Chem. 57, 13470 (2018). https://doi.org/10.1021/acs.inorgchem.8b02087
D. A. Shultz, R. Stephenson, and M. L. Kirk, Dalton Trans. 52, 1970 (2023). https://doi.org/10.1039/D2DT03385B
P. A. Scattergood, P. Jesus, H. Adams, et al., Dalton Trans. 44, 11705 (2015). https://doi.org/10.1039/C4DT03466J
M. P. Bubnov, A. V. Piskunov, A. A. Zolotukhin, et al., Russ. J. Coord. Chem. 46, 224 (2020). https://doi.org/10.1134/S107032842003001X
T. N. Kocherova, N. O. Druzhkov, M. V. Arsenyev, et al., Russ. Chem. Bull. 72, 1192 (2023). https://doi.org/10.1007/s11172-023-3889-8
T. N. Kocherova, N. O. Druzhkov, K. A. Martyanov, et al., Rus. Chem. Bull. 69, 2383 (2020). https://doi.org/10.1007/s11172-020-3051-9
T. N. Kocherova, N. O. Druzhkov, A. S. Shavyrin, et al., Russ. Chem. Bull. 70, 916 (2021). https://doi.org/10.1007/s11172-021-3167-6
A. V. Maleeva, O. Yu. Trofimova, T. N. Kocherova, et al., Russ. J. Coord. Chem. 49, 718 (2023). https://doi.org/10.1134/S1070328423600742
A. V. Klimashevskaya, K. V. Arsenyeva, A. V. Cherkasov, et al., J. Struct. Chem. 64, 2271 (2023). https://doi.org/10.1134/S0022476623120016
S. V. Baryshnikova, M. V. Arsen’ev, R. V. Rumyantsev, et al., Russ. J. Coord. Chem. 49, 429 (2023). https://doi.org/10.1134/S107032842360016X
E. B. Van der Tol, H. J. Van Ramesdonk, J. W. Verhoeven, et al., Chem.—Eur. J. 4, 2315 (1998). https://doi.org/10.1002/(SICI)1521-3765(19981102)4:1-1<2315::AID-CHEM2315>3.0.CO;2-E
Rigaku Oxford Diffraction C.s.s., ver. 1.171.41.39a. Rigaku Corporation, Wroclaw, Poland, 2020.
G. M. Sheldrick, Acta Crystallogr. C71, 3 (2015). https://doi.org/10.1107/S2053229614024218
G. M. Sheldrick, Acta Crystallogr. A71, 3 (2015). https://doi.org/10.1107/S2053273314026370
R. D. Svetogorov, P. V. Dorovatovskii, and V. A. Lazarenko, Cryst. Res. Technol. 55, 1900184 (2020). https://doi.org/10.1002/crat.201900184
W. Kabsch, Acta Crystallogr., Sect. D 66, 125 (2010). https://doi.org/10.1107/S0907444909047337
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009). https://doi.org/10.1107/S0021889808042726
V. I. Ovcharenko, E. V. Gorelik, S. V. Fokin, et al., J. Am. Chem. Soc. 129, 10512 (2007). https://doi.org/10.1021/ja072463b
S. L. Veber, M. V. Fedin, S. V. Fokin, et al., Appl. Magn. Reson. 37, 693 (2010). https://doi.org/10.1007/s00723-009-0087-2
J. S. Thompson and J. C. Calabrese, J. Am. Chem. Soc. 108, 1903 (1986). https://doi.org/10.1021/ja00268a031
S. N. Brown, Inorg. Chem. 51, 1251 (2012). https://doi.org/10.1021/ic202764j
B. J. Hathaway and D. E. Billing, Coord. Chem. Rev. 5, 143 (1970). https://doi.org/10.1016/S0010-8545(00)80135-6
A. V. Piskunov, A. V. Maleeva, I. N. Mescheryakova, et al., Eur. J. Inorg. Chem. 4318 (2012). https://doi.org/10.1002/ejic.201200535
M. G. Chegerev, A. V. Piskunov, A. V. Maleeva, et al., Eur. J. Inorg. Chem. 2016, 3813 (2016). https://doi.org/10.1002/ejic.201600501
R. A. Davidson, J. Hao, A. L. Rheingold, et al., Polyhedron 136, 176 (2017). https://doi.org/10.1016/j.poly.2017.10.003
M. A. Zherebtsov, M. V. Arsenyev, E. V. Baranov, et al., J. Struct. Chem. 64, 2051 (2023). https://doi.org/10.1134/S0022476623110033
P. Verma, J. Weir, L. Mirica, et al., Inorg. Chem. 50, 9816 (2011). https://doi.org/10.1021/ic200958g
S. S. Batsanov, Russ. J. Inorg. Chem. 36, 1694 (1991).
O. Yu. Trofimova, K. I. Pashanova, I. V. Ershova, et al., Russ. J. Inorg. Chem. 68, 1166 (2023). https://doi.org/10.1134/S0036023623601344
C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, 2nd Ed. (Wiley-VCH, 1988).
ACKNOWLEDGMENTS
The work was carried out using the equipment of the Center for Collective Use “Analytical Center of the G. A. Razuvaev Institute of Organometallic Chemistry of the Russian Academy of Sciences” (Nizhny Novgorod, Russia). X-ray diffraction data for complex II were obtained at the X-ray beam of the Belok station of the Kurchatov Synchrotron Radiation Center at the National Research Center “Kurchatov Institute” (Moscow, Russia).
Funding
This study was carried out within the framework of the State assignment of the G. A. Razuvaev Institute of Organometallic Chemistry of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trofimova, O.Y., Maleeva, A.V., Arseniev, M.V. et al. Copper(II) Complexes with Mono- and Double Reduced Forms of 3,5-Di-tert-octyl-o-benzoquinone. Russ. J. Inorg. Chem. (2024). https://doi.org/10.1134/S0036023623602945
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0036023623602945