Abstract
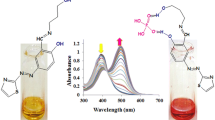
Reactions of pyridine (Py) with (5,10,15,20-(tetra-4-tert-butylphenyl)porphinato)trichloroniobium(V) (Nb(Cl)3TtBuPP) and H+-bonded Nb(Cl)3TtBuPP (Nb(Cl)3TtBuPP···H+···Cl−) in toluene have been studied using spectroscopy (UV-vis, IR, 1H NMR, mass spectrometry, fluorescence), thermodynamics and kinetics. The process is a system of consecutive two- and one-way reactions of the two pyridine molecules bonding; the nature of this interactions is determined by the chemical structure of the initial niobium(V) porphyrin. The reactions have been completely quantitatively described, and the intermediate and final products spectral parameters used for the product chemical structure confirmation have been determined. It has been demonstrated that Nb(Cl)3TtBuPP and Nb(Cl)3TtBuPP···H+···Cl− are good candidates for use as optical and fluorescent chemosensors of VOCs (volatile organic compounds) and nitrogenous bases—building blocks of pharmaceuticals, food components, and environmental pollutants—with the following parameters: the stability constant of the complex with pyridine K = (1.99 ± 0.3) × 104 L2/mol2 and (2.8 ± 0.5) × 102 L/mol, relative optical response A = 0.91 and 0.35, detection limit of Py 1.74 × 10–3 and 4.05 × 10–4 mol/L, respectively. The results are applicable for use in the design of dye-sensitized solar cells (DSSCs) since the reaction studied is a model for self-assembly of donor–acceptor systems based on metalloprorphyrins and pyridyl derivatives of carbon nanoforms.










Similar content being viewed by others
REFERENCES
M. Ziolek and I. Sobczak, Catal. Today 285, 211 (2017). https://doi.org/10.1016/j.cattod.2016.12.013
C. J. Carmalt, C. W. Dinnage, I. P. Parkin, et al., Inorg. Chem. 41, 3668 (2002). https://doi.org/10.1021/ic020097l
U. J. Kilgore, J. Tomaszewski, H. Fan, et al., Organometallics 26, 6132 (2007). https://doi.org/10.1021/om7008233
T. Matsuo and H. Kawaguchi, Inorg. Chem. 41, 6090 (2002). https://doi.org/10.1021/ic025882c
D. Bayot and M. Devillers, Coord. Chem. Rev. 250, 2610 (2006). https://doi.org/10.1016/j.ccr.2006.04.011
R. W. Berg, Coord. Chem. Rev. 113, 1 (1992).
T. Poursaberi, M. R. Ganjali, and M. Hassanisadi, Talanta 101, 128 (2012).
Yuan Lv-Bing, He Yan-**, Zhang Lei, et al., Inorg. Chem. 57, 4226 (2018). https://doi.org/10.1021/acs.inorgchem.7b03265
G. R. Morello and T. R. Cundari, Organometallics 35, 3624 (2016). https://doi.org/10.1021/acs.organomet.6b00679
J. E. Anderson, Y. H. Liu, R. Guilard, et al., Inorg. Chem. 25, 3786 (1986). https://doi.org/10.1021/ic00241a017
E. W. Y. Wong, C. J. Walsby, T. Storr, et al., Inorg. Chem. 49, 3343 (2010). https://doi.org/10.1021/ic902409n
Lee. Hosoowi, Hong. Kyeong-Im, and Jang. Woo-Dong, Coord. Chem. Rev. 354, 46 (2018). https://doi.org/10.1016/j.ccr.2017.06.008
J. F. Callan, A. P. de Silva, and D. C. Magri, Tetrahedron 61, 8551 (2005).
Tiening **, Junchao Zhou, Hao-Yu Greg Lin, et al., Anal. Chem. 91, 817 (2019). https://doi.org/10.1021/acs.analchem.8b03004
A. Colombelli, M. G. Manera, and V. Borovkov, Sens. Actuators B: Chem. 246, 1039 (2017). https://doi.org/10.1016/j.snb.2017.01.192
T. N. Lomova, M. E. Klyueva, and B. D. Berezin, Russ. J. Chem. Chem. Technol. 31, 75 (1988).
M. Y. Tipugina and T. N. Lomova, Russ. J. Phys. Chem. 76, 567 (2002).
T. N. Lomova, E. V. Motorina, E. N. Ovchenkova, et al., Russ. Chem. Bull. 56, 660 (2007). https://doi.org/10.1007/s11172-007-0105-1
E. V. Motorina, E. G. Mozhzhukhina, and T. N. Lomova, J. Struct. Chem. 59, 1880 (2018). https://doi.org/10.1134/S0022476618080164
E. V. Motorina, T. N. Lomova, and M. V. Klyuev, Mendeleev Commun. 28, 426 (2018). https://doi.org/10.1016/j.mencom.2018.07.029
Y. Matsuda and Y. Murakami, Coord. Chem. Rev. 92, 157 (1988).
T. N. Lomova, Theoretical and Experimental Methods of Solution Chemistry (Problems of Solution Chemistry) (Prospekt, Moscow, 2011) [in Russian].
T. N. Lomova, Axially Coordinated Metalloporphyrins in Science and Practice (Krasand, Mooscow, 2018) [in Russian].
G. F. Bol’shakov and E. A. Glebovskaya, Tables of Infrared Frequencies of Heteroorganic Compounds (Khimiya, Leningrad, 1968) [in Russian].
A. V. Garmash and N. M. Sorokina, Metrological Foundations of Analytical Chemistry (Moscow, 2017) [in Russian].
V. I. Dvorkin, Metrology and Quality Assurance of Quantitative Chemical Analysis (Khimiya, Moscow, 2001) [in Russian].
Electronic Resource, Limit of Detection, Limit of Quantification, and Analyte Concentration Limits. https://studref.com/504076/matematika_himiya_fizik/ predel_obnaruzheniya_predel_opredeleniya_graniny_opredelyaemyh_soderzhaniy
A. D. Dubonosov, A. V. Tsukanov, I. E. Tolpygin, et al., Bull. South. Sci. Center 9, 70 (2013).
ACKNOWLEDGMENTS
We are grateful to N.G. Bichan and Yu.A. Gubarev for their help with the experiment.
The physicochemical experiment was performed on the equipment of the Upper Volga Regional Center for Physicochemical Research.
Funding
This work was carried out in accordance with the Program of State Academies of Sciences (topic no. 0092-2014-0002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Motorina, E.V., Lomova, T.N., Mozhzhukhina, E.G. et al. New Molecular Chemosensors Based on Niobium(V) 5,10,15,20-(Tetra-4-tert-butylphenyl)porphine for Detection of VOCs. Russ. J. Inorg. Chem. 64, 1538–1547 (2019). https://doi.org/10.1134/S0036023619120106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619120106