Abstract
Pathogenesis of the novel coronavirus infection COVID-19 is the subject of active research around the world. COVID-19 caused by the SARS-CoV-2 is a complex disease in which interaction of the virus with target cells, action of the immune system and the body’s systemic response to these events are closely intertwined. Many respiratory viral infections, including COVID-19, cause death of the infected cells, activation of innate immune response, and secretion of inflammatory cytokines. All these processes are associated with the development of oxidative stress, which makes an important contribution to pathogenesis of the viral infections. This review analyzes information on the oxidative stress associated with the infections caused by SARS-CoV-2 and other respiratory viruses. The review also focuses on involvement of the vascular endothelium in the COVID-19 pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the majority of cases, the novel coronavirus SARS-CoV-2 causes respiratory disease requiring no special medical intervention, but up to 20% of COVID-19 patients require hospitalization [1]. Severe COVID-19 infection triggers imbalanced and uncontrolled cytokine response (called cytokine storm), exuberant endothelial inflammatory reactions, and vascular thrombosis. These and probably other, yet unknown factors may lead to the development of acute respiratory distress syndrome (ARDS), a major cause of death of the COVID-19 patients [90] demonstrated that pulmonary angiogenesis during COVID-19 was increased by 2.7-fold in comparison with the one in patients died from consequences of the influenza virus A infection. It was suggested that the mechanism behind this phenomenon was based on activated intussusceptive angiogenesis typical to normal development, wound healing, and diverse pathologies [91]. During COVID-19, angiogenesis seems to result from the endothelium damage and hypoxia within the lung injury foci. Elevated ACE2 levels may be related to enhanced angiogenesis or its compensatory expression occurring after virus-mediated blockade of its enzymatic activity, which awaits further investigation (hereinafter the data available in open access on July 20, 2020 are presented). It should be noted that the direct virus-related endothelium damage is a crucial component in pathogenesis of the influenza virus infection as well [92], but intensity of this damage is profoundly lower and it rarely caused severe consequences in comparison with the infection caused by SARS-CoV-2.
Interaction of the lung capillary endothelium with SARS-CoV-2 could occur at early infection stage presuming that potentially it might exit into the bloodstream without disrupting alveolar epithelial cells in close proximity to the capillary endothelium. In the later stages of infection, massive release of the viruses to the blood flow from the large number of destroyed alveolar cells could cause infection of endothelial cells in other vessels. Even without invading sensitive cells, virus may induce some endothelial response by binding to ACE2 and suppressing its proteolytic activity. ACE2 (zinc metalloprotease) cleaves peptide hormone angiotensin-II (ATII), which exerts multiple functions including inducing vasoconstriction causing high blood pressure. Peptides formed due to ATII cleavage may stimulate signaling counteracting ATII. While ACE2 activity declines after binding to viral S-protein, ATII level may be markedly elevated in pulmonary capillaries. Local accumulation of ATII in the lungs was experimentally demonstrated in the bleomycin-induced pulmonary fibrosis model [93]. In this model alveolar epithelial cells and pulmonary myofibroblasts were the main sources of ATII. Systemically elevated ATII in the blood flow during COVID19 seems highly unlikely because high viremia has not been observed even in the severe disease forms, whereas ACE2 is an ubiquitous protein. Up to now, the elevated serum ATII level during COVID-19 infection was reported only in a single study [94].
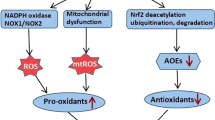
AT1R serves as a major ATII receptor that activates multilayered signaling in endothelial cells including MAP-kinase axis, protein kinase C, as well as transcription factor NF-κB resulting in activation of NOX2, expression of cytokines, adhesion molecules, and cyclooxygenase 2 (COX2) [95]. Endothelial NOX2 acts as a major source of ROS production, which turned out to be required for AT1R downstream signaling [96]. It was found by Dikalov et al. [97, 98] that stimulation of ATII resulted in the elevated mtROS production that further enhanced NOX2 activity. Experiments with mitochondrial inhibitors and mitochondria-targeted antioxidants revealed that the reduced mtROS production suppressed AT1R signaling. The question regarding the cause of the increased mtROS production still remains open. Dikalov et al. demonstrated that the ATII-induced signaling was lower in the cyclophilin D (CypD) knockout mice [99]. This mitochondrial protein serves as a regulatory component of the mitochondrial permeability transition pore (mPTP), opening of which may result in the increased mtROS level [100]. ROS produced by NOX2 may be one of the causes for pore opening. Thus, ATII initiates positive feedback loop leading to oxidative stress and endothelial dysfunction (figure).
Angiotensin II (ATII) interacts with AT1R receptor and induces ROS production via NADPH oxidase (NOX) in endothelial cells triggering mitochondrial oxidative stress and endothelial dysfunction. It is believed that SARS-CoV-2 S-protein binds to ACE2 causing its subsequent local or systemic depletion of this enzyme that cleaves ATII, which, in turn, increased ATII level.
ATII-induced endothelial activation may occur cooperatively with pro-inflammatory cytokines. In particular, IL-6 stimulates AT1R expression and ATII-dependent signaling that result in the further enhancement of oxidative stress and endothelial dysfunction [101]. IL6 knockout mice exhibit lower endothelial dysfunction caused by administration of ATII [73].
Furthermore, in the acute lung failure model caused by acid aspiration or with bacterial wall lipopolysaccharide ACE2 knockout mice exhibited significantly more substantial tissue damage, whereas recombinant ACE2 or AT1R inhibitor protected from lung injury [102]. Upregulated expression of adhesion molecules, increased secretion of pro-inflammatory cytokines and chemokines, as well as elevated permeability result in acute inflammatory reaction in endothelium in the case of increase of the normal ATII level. Moreover, it is likely to enhance platelet deposition and release of the von Willebrand blood clotting factor that may account for one of causes of develo** thrombosis [103]. Under normal conditions, impact of ATII on thrombogenesis seems to be insignificant [104], but during COVID-19 such effect could be likely. In particular, the pro-inflammatory cytokines may activate platelets in COVID-19 patients that presumably promotes thrombogenesis [105].
Along with endothelial activation, ATII causes secretion of the pro-inflammatory cytokines in alveolar epithelium [106] and changes in the alveolar fluid clearance associated with inactivation of Na+-channels [107]. Moreover, ATII induces alveolar epithelial-mesenchymal transition that causes enhanced epithelial permeability and pulmonary edema [108]. Finally, high ATII levels trigger epithelial cell apoptosis [109].
It should be noted that the hypothesized role of ATII in COVID-19 pathogenesis has been proposed repeatedly (e.g., see [110]), but, however, has not been confirmed experimentally. Drugs blocking ATII production or AT1R signaling are commonly used in treating blood hypertension. Large-scale study conducted by the New York University (NYU) Grossman School of Medicine [111] revealed that taking these drugs does not affect likelihood of infection or risk of severe COVID-19. Using recombinant soluble ACE2 in vitro substantially lowered infection with SARS-CoV-2 owing to competition with the natural ACE2 for virus binding [112]. It can be suggested that such protein would lower ATII level and prevent develo** COVID-19 infection.
At present, no experimental data allowing to assess effects of infection of endothelial cells with SARS-CoV-2 have been obtained. Studies examining interactions between the SARS-CoV-1 and epithelial cells started in 2004 [113] were put on hold. No this type of studies were conducted for the MERS coronavirus. Only few studies aimed at investigating impaired endothelial function after infection with influenza A virus have been reported. In particular, it was shown that this virus in murine model lowered the level of endothelial Krüppel-like Factor 2 (KLF2) [114]. This factor restricts inflammatory activation of endothelium, prevents disruption of permeability and development of atherosclerosis [115]. Interestingly, that Nrf2 is one of the targets of KLF2 in endothelium [116], therefore it is likely that its activation explains protective effects of KLF2.
PERSPECTIVES FOR ANTIOXIDANT THERAPY IN COVID-19
Currently, there are multiple trials underway that test antioxidants as therapeutic agents in COVID-19 (https://clinicaltrials.gov/), but no results were available at the time of preparing current review. Nonetheless, antioxidants such NAC have been already included into the clinical protocols for treating moderate and severe COVID-19 [117].
Most notably, use of antioxidants seems reasonable at the stage requiring inhibition of inflammatory reactions during COVID-19. It is expected that such therapy may prevent organ and tissue damage due to cytokine storm and oxidative stress [118, 119].
Furthermore, lowering oxidative stress by antioxidants may result in the decreased viral load. Recent study with peripheral blood monocytes purified from the healthy volunteers demonstrated that SARS-CoV-2 replication was suppressed by NAC and mitochondria-targeted antioxidant MitoQ [48] allowing to assume that the decreased ROS levels prevent HIF1-α activation and subsequent metabolic switch to glycolysis necessary for coronavirus replication. However, this hypothesis requires further investigation.
Furthermore, To et al. [120] proposed another way of using mitochondria-targeted antioxidant MitoTEMPO in the their study investigating its preventive and therapeutic action in murine model of H3N2 influenza virus infection. In particular, they found that intranasally administered MitoTEMPO decreased mouse mortality, virus titer, as well as lowered airway tract inflammation and decreased neutrophil infiltration. Precise antiviral mechanisms exerted by MitoTEMPO remain obscure; it is likely that the decrease of mtROS results in downregulated expression of the cell-cell adherens junctions, which explains decreased immune cell infiltration as well as reduced activity of NLRP3 inflammasome that produces IL-1β. The decline in virus titer could be explained by the elevated amount of antiviral interferon IFN-1β, which, however, was assessed solely at mRNA rather than protein level. It must be mentioned that the use of MitoTEMPO did not compromise adaptive immune response induced by pulmonary dendritic cells and did not affect population of the lung B and T cell involved in humoral and cellular immunity [120].
Potentially, antioxidants may influence thrombogenesis, which is a common and dangerous complication during COVID-19. Cytokine storm may result in the ROS-dependent apoptosis in endothelial cells [121], whereas SkQ1-mediated mtROS decline prevents TNF-induced apoptosis in vitro [65]. Lowering endothelial cell death could also prevent activation of thrombogenesis.
Another approach in the fight against oxidative stress during COVID-19 involves induction of endogenous antioxidant systems. For instance, the transcription factor Nrf2 controls expression of antioxidant and other cell defense systems. Experiments with mice treated with ATII for 14 days demonstrated that Nrf2 activation with the help of tert-butylhydroquinone lowered ROS level as well as decreased microvascular endothelial dysfunction and hypertension [122]. Similar data were also obtained in vitro in small artery-derived endothelial cells by activating Nrf2 with sulforaphane [123]. The use of Nrf2 activators for COVID-19 therapy is discussed in more detail elsewhere [124].
Thus, the use of mitochondria-targeted antioxidants looks as a promising approach to lower oxidative stress and accompanying complications in viral infections. Further experiments with animal models and clinical trials are necessary to reveal therapeutic potential for such approach.
Abbreviations
- ARDS:
-
acute respiratory distress syndrome
- ATII:
-
angiotensin II
- ROS:
-
reactive oxygen species
- mtROS:
-
mitochondrial reactive oxygen species
- NOX:
-
NADPH oxidase
References
Wu, Z., and McGoogan, J. M. (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention, JAMA, 323, 1239-1242, https://doi.org/10.1001/jama.2020.2648.
Wu, C., Chen, X., Cai, Y., **a, J., Zhou, X., et al. (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Intern. Med., 180, 934-943, https://doi.org/10.1001/jamainternmed.2020.0994.
Varga, Z., Flammer, A. J., Steiger, P., Haberecker, M., Andermatt, R., et al. (2020) Endothelial cell infection and endotheliitis in COVID-19, Lancet, 395, 1417-1418, https://doi.org/10.1016/S0140-6736(20)30937-5.
Blanco-Melo, D., Nilsson-Payant, B. E., Liu, W.-C., Uhl, S., Hoagland, D., et al. (2020) Imbalanced host response to SARS-CoV-2 drives development of COVID-19, Cell, 181, 1036-1045.e9, https://doi.org/10.1016/j.cell.2020.04.026.
Peterhans, E. (1979) Sendai virus stimulates chemiluminescence in mouse spleen cells, Biochem Biophys. Res. Commun., 91, 383-392, https://doi.org/10.1016/0006-291x(79)90630-2.
Khomich, O. A., Kochetkov, S. N., Bartosch, B., and Ivanov, A. V. (2018) Redox biology of respiratory viral infections, Viruses, 10, 392, https://doi.org/10.3390/v10080392.
Buffinton, G. D., Christen, S., Peterhans, E., and Stocker, R. (1992) Oxidative stress in lungs of mice infected with influenza A virus, Free Radic Res. Commun., 16, 99-110, https://doi.org/10.3109/10715769209049163.
Amatore, D., Sgarbanti, R., Aquilano, K., Baldelli, S., Limongi, D., et al. (2015) Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS, Cell Microbiol., 17, 131-145, https://doi.org/10.1111/cmi.12343.
Hendricks, K., To, E., Vlahos, R., Broughton, B., Peshavariya, H., and Selemidis, S. (2016) Influenza A virus causes vascular endothelial cell oxidative stress via NOX2 oxidase, Eur. Respir. J., 48, PA3967, https://doi.org/10.1183/13993003.congress-2016.PA3967.
Martínez, I., García-Carpizo, V., Guijarro, T., García-Gomez, A., Navarro, D., Aranda, A., and Zambrano, A. (2016) Induction of DNA double-strand breaks and cellular senescence by human respiratory syncytial virus, Virulence, 7, 427-442, https://doi.org/10.1080/21505594.2016.1144001.
Biagioli, M. C., Kaul, P., Singh, I., and Turner, R. B. (1999) The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells, Free Radic. Biol. Med., 26, 454-462, https://doi.org/10.1016/s0891-5849(98)00233-0.
Lim, J.-Y., Oh, E., Kim, Y., Jung, W.-W., Kim, H.-S., Lee, J., and Sul, D. (2014) Enhanced oxidative damage to DNA, lipids, and proteins and levels of some antioxidant enzymes, cytokines, and heat shock proteins in patients infected with influenza H1N1 virus, Acta Virol., 58, 253-260, https://doi.org/10.4149/av_2014_03_253.
Erkekoğlu, P., Aşçı, A., Ceyhan, M., Kızılgün, M., Schweizer, U., et al. (2013) Selenium levels, selenoenzyme activities and oxidant/antioxidant parameters in H1N1-infected children, Turk. J. Pediatr., 55, 271-282.
Ng, M. P. E., Lee, J. C. Y., Loke, W. M., Yeo, L. L. L., Quek, A. M. L., et al. (2014) Does influenza A infection increase oxidative damage? Antioxid. Redox Signal., 21, 1025-1031, https://doi.org/10.1089/ars.2014.5907.
Nin, N., Sánchez-Rodríguez, C., Ver, L. S., Cardinal, P., Ferruelo, A., et al. (2012) Lung histopathological findings in fatal pandemic influenza A (H1N1), Med. Intensiva, 36, 24-31, https://doi.org/10.1016/j.medin.2011.10.005.
Reshi, M. L., Su, Y.-C., and Hong, J.-R. (2014) RNA viruses: ROS-mediated cell death, Int. J. Cell. Biol., 2014, 467452, https://doi.org/10.1155/2014/467452.
Finkel, T. (2011) Signal transduction by reactive oxygen species, J. Cell. Biol., 194, 7-15, https://doi.org/10.1083/jcb.201102095.
Yang, Y., Bazhin, A. V., Werner, J., and Karakhanova, S. (2013) Reactive oxygen species in the immune system, Int. Rev. Immunol., 32, 249-270, https://doi.org/10.3109/08830185.2012.755176.
To, E. E., Broughton, B. R. S., Hendricks, K. S., Vlahos, R., and Selemidis, S. (2014) Influenza A virus and TLR7 activation potentiate NOX2 oxidase-dependent ROS production in macrophages, Free Radic. Res., 48, 940-947, https://doi.org/10.3109/10715762.2014.927579.
Kaul, P., Biagioli, M. C., Singh, I., and Turner, R. B. (2000) Rhinovirus-induced oxidative stress and interleukin-8 elaboration involves p47-phox but is independent of attachment to intercellular adhesion molecule-1 and viral replication, J. Infect. Dis., 181, 1885-1890, https://doi.org/10.1086/315504.
Fink, K., Duval, A., Martel, A., Soucy-Faulkner, A., and Grandvaux, N. (2008) Dual role of NOX2 in respiratory syncytial virus- and sendai virus-induced activation of NF-kappaB in airway epithelial cells, J. Immunol., 180, 6911-6922, https://doi.org/10.4049/jimmunol.180.10.6911.
Ye, S., Lowther, S., and Stambas, J. (2015) Inhibition of reactive oxygen species production ameliorates inflammation induced by influenza A viruses via upregulation of SOCS1 and SOCS3, J. Virol., 89, 2672-2683, https://doi.org/10.1128/JVI.03529-14.
Vlahos, R., Stambas, J., Bozinovski, S., Broughton, B. R. S., Drummond, G. R., and Selemidis, S. (2011) Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation, PLoS Pathog., 7, e1001271, https://doi.org/10.1371/journal.ppat.1001271.
Snelgrove, R. J., Edwards, L., Rae, A. J., and Hussell, T. (2006) An absence of reactive oxygen species improves the resolution of lung influenza infection, Eur. J. Immunol., 36, 1364-1373, https://doi.org/10.1002/eji.200635977.
Turrens, J. F. (2003) Mitochondrial formation of reactive oxygen species, J. Physiol., 552, 335-344, https://doi.org/10.1113/jphysiol.2003.049478.
Vorobjeva, N., Prikhodko, A., Galkin, I., Pletjushkina, O., Zinovkin, R., et al. (2017) Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro, Eur. J. Cell. Biol., 96, 254-265, https://doi.org/10.1016/j.ejcb.2017.03.003.
Zinovkin, R. A., Romaschenko, V. P., Galkin, I. I., Zakharova, V. V., Pletjushkina, O. Y., Chernyak, B. V., and Popova, E. N. (2014) Role of mitochondrial reactive oxygen species in age-related inflammatory activation of endothelium, Aging, 6, 661-674, https://doi.org/10.18632/aging.100685.
Zhou, R., Yazdi, A. S., Menu, P., and Tschopp, J. (2011) A role for mitochondria in NLRP3 inflammasome activation, Nature, 469, 221-225, https://doi.org/10.1038/nature09663.
Williamson, E. J., Walker, A. J., Bhaskaran, K., Bacon, S., Bates, C., et al. (2020) OpenSAFELY: factors associated with COVID-19 death in 17 million patients, Nature, 584, 430-436, https://doi.org/10.1038/s41586-020-2521-4.
Skulachev, V. P., Anisimov, V. N., Antonenko, Y. N., Bakeeva, L. E., Chernyak, B. V., et al. (2009) An attempt to prevent senescence: a mitochondrial approach, Biochim. Biophys. Acta, 1787, 437-461, https://doi.org/10.1016/j.bbabio.2008.12.008.
Morris, A. A., Zhao, L., Patel, R. S., Jones, D. P., Ahmed, Y., et al. (2012) Differences in systemic oxidative stress based on race and the metabolic syndrome: the morehouse and emory team up to eliminate health disparities (meta-health) study, Metab. Syndr. Relat. Disord., 10, 252-259, https://doi.org/10.1089/met.2011.0117.
Kander, M. C., Cui, Y., and Liu, Z. (2017) Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases, J. Cell. Mol. Med., 21, 1024-1032, https://doi.org/10.1111/jcmm.13038.
Janicki-Deverts, D., Cohen, S., Matthews, K. A., Gross, M. D., and Jacobs, D. R., Jr. (2009) Socioeconomic status, antioxidant micronutrients, and correlates of oxidative damage: the coronary artery risk development in young adults (CARDIA) study, Psychosom. Med., 71, 541-548, https://doi.org/10.1097/PSY.0b013e31819e7526.
King, G. L., and Loeken, M. R. (2004) Hyperglycemia-induced oxidative stress in diabetic complications, Histochem. Cell. Biol., 122, 333-338, https://doi.org/10.1007/s00418-004-0678-9.
Delgado-Roche, L., and Mesta, F. (2020) Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection, Arch. Med. Res., 51, 384-387, https://doi.org/10.1016/j.arcmed.2020.04.019.
Massaro, G. D., Gail, D. B., and Massaro, D. (1975) Lung oxygen consumption and mitochondria of alveolar epithelial and endothelial cells, J. Appl. Physiol., 38, 588-592, https://doi.org/10.1152/jappl.1975.38.4.588.
Cloonan, S. M., and Choi, A. M. K. (2016) Mitochondria in lung disease, J. Clin. Invest., 126, 809-820, https://doi.org/10.1172/JCI81113.
Park, H. S., Kim, S. R., and Lee, Y. C. (2009) Impact of oxidative stress on lung diseases, Respirology, 14, 27-38, https://doi.org/10.1111/j.1440-1843.2008.01447.x.
Mach, W. J., Thimmesch, A. R., Pierce, J. T., and Pierce, J. D. (2011) Consequences of hyperoxia and the toxicity of oxygen in the lung, Nurs. Res. Pract., 2011, 260482, https://doi.org/10.1155/2011/260482.
Das, K. C. (2013) Hyperoxia decreases glycolytic capacity, glycolytic reserve and oxidative phosphorylation in MLE-12 cells and inhibits complex I and II function, but not complex IV in isolated mouse lung mitochondria, PLoS One, 8, e73358, https://doi.org/10.1371/journal.pone.0073358.
Merad, M., and Martin, J. C. (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages, Nat. Rev. Immunol., 20, 355-362, https://doi.org/10.1038/s41577-020-0331-4.
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., Manson, J. J., and HLH Across Speciality Collaboration, UK (2020) COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet, 395, 1033-1034, https://doi.org/10.1016/S0140-6736(20)30628-0.
Zhang, W., Zhao, Y., Zhang, F., Wang, Q., Li, T., et al. (2020) The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China, Clin. Immunol., 214, 108393, https://doi.org/10.1016/j.clim.2020.108393.
Chen, G., Wu, D., Guo, W., Cao, Y., Huang, D., et al. (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019, J. Clin. Invest., 130, 2620-2629, https://doi.org/10.1172/JCI137244.
Chen, I.-Y., Moriyama, M., Chang, M.-F., and Ichinohe, T. (2019) Severe acute respiratory syndrome coronavirus Viroporin 3a activates the NLRP3 inflammasome, Front. Microbiol., 10, 50, https://doi.org/10.3389/fmicb.2019.00050.
Xu, J., Zhao, S., Teng, T., Abdalla, A. E., Zhu, W., **e, L., Wang, Y., and Guo, X. (2020) Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV, Viruses, 12, 244, https://doi.org/10.3390/v12020244.
Hoepel, W., Chen, H.-J., Allahverdiyeva, S., Manz, X., Aman, J., et al. (2020) Anti-SARS-CoV-2 IgG from severely ill COVID-19 patients promotes macrophage hyper-inflammatory responses, bioRxiv, https://doi.org/10.1101/2020.07.13.190140.
Codo, A. C., Davanzo, G. G., de Brito Monteiro, L., de Souza, G. F., Muraro, S. P., et al. (2020) Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis, Cell. Metab., 32, 437-446.e5, https://doi.org/10.1016/j.cmet.2020.07.007.
Teijaro, J. R., Walsh, K. B., Cahalan, S., Fremgen, D. M., Roberts, E., et al. (2011) Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection, Cell, 146, 980-991, https://doi.org/10.1016/j.cell.2011.08.015.
Herwig, M. C., Tsokos, M., Hermanns, M. I., Kirkpatrick, C. J., and Müller, A. M. (2013) Vascular endothelial cadherin expression in lung specimens of patients with sepsis-induced acute respiratory distress syndrome and endothelial cell cultures, Pathobiology, 80, 245-251, https://doi.org/10.1159/000347062.
Dreymueller, D., Pruessmeyer, J., Groth, E., and Ludwig, A. (2012) The role of ADAM-mediated shedding in vascular biology, Eur. J. Cell. Biol., 91, 472-485, https://doi.org/10.1016/j.ejcb.2011.09.003.
Angelini, D. J., Hyun, S.-W., Grigoryev, D. N., Garg, P., Gong, P., et al. (2006) TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia, Am. J. Physiol. Lung Cell. Mol. Physiol., 291, L1232-L1245, https://doi.org/10.1152/ajplung.00109.2006.
Marcos-Ramiro, B., García-Weber, D., and Millán, J. (2014) TNF-induced endothelial barrier disruption: beyond actin and Rho, Thromb. Haemost., 112, 1088-1102, https://doi.org/10.1160/TH14-04-0299.
Sawant, D. A., Wilson, R. L., Tharakan, B., Stagg, H. W., Hunter, F. A., and Childs, E. W. (2014) Tumor necrosis factor-α-induced microvascular endothelial cell hyperpermeability: role of intrinsic apoptotic signaling, J. Physiol. Biochem., 70, 971-980, https://doi.org/10.1007/s13105-014-0366-8.
Sarelius, I. H., and Glading, A. J. (2015) Control of vascular permeability by adhesion molecules, Tissue Barriers, 3, e985954, https://doi.org/10.4161/21688370.2014.985954.
Schmidt, E. P., Yang, Y., Janssen, W. J., Gandjeva, A., Perez, M. J., et al. (2012) The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis, Nat. Med., 18, 1217-1223, https://doi.org/10.1038/nm.2843.
Parks, W. C., Wilson, C. L., and López-Boado, Y. S. (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity, Nat. Rev. Immunol., 4, 617-629, https://doi.org/10.1038/nri1418.
Galkin, I. I., Pletjushkina, O. Y., Zinovkin, R. A., Zakharova, V. V., Chernyak, B. V., and Popova, E. N. (2016) Mitochondria-targeted antioxidant SkQR1 reduces TNF-induced endothelial permeability in vitro, Biochemistry (Moscow), 81, 1188-1197, https://doi.org/10.1134/S0006297916100163.
Romaschenko, V. P., Zinovkin, R. A., Galkin, I. I., Zakharova, V. V., Panteleeva, A. A., et al. (2015) Low concentrations of uncouplers of oxidative phosphorylation prevent inflammatory activation of endothelial cells by tumor necrosis factor, Biochemistry (Moscow), 80, 610-619, https://doi.org/10.1134/S0006297915050144.
Zakharova, V. V., Pletjushkina, O. Y., Galkin, I. I., Zinovkin, R. A., Chernyak, B. V., et al. (2017) Low concentration of uncouplers of oxidative phosphorylation decreases the TNF-induced endothelial permeability and lethality in mice, Biochim. Biophys. Acta Mol. Basis Dis., 1863, 968-977, https://doi.org/10.1016/j.bbadis.2017.01.024.
Mukherjee, T. K., Mukhopadhyay, S., and Hoidal, J. R. (2005) The role of reactive oxygen species in TNFalpha-dependent expression of the receptor for advanced glycation end products in human umbilical vein endothelial cells, Biochim. Biophys. Acta, 1744, 213-223, https://doi.org/10.1016/j.bbamcr.2005.03.007.
Min, J.-K., Kim, Y.-M., Kim, S. W., Kwon, M.-C., Kong, Y.-Y., et al. (2005) TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells, J. Immunol., 175, 531-540, https://doi.org/10.4049/jimmunol.175.1.531.
Spindler, V., Schlegel, N., and Waschke, J. (2010) Role of GTPases in control of microvascular permeability, Cardiovasc. Res., 87, 243-253, https://doi.org/10.1093/cvr/cvq086.
Van Wetering, S., van Buul, J. D., Quik, S., Mul, F. P. J., Anthony, E. C., et al. (2002) Reactive oxygen species mediate Rac-induced loss of cell–cell adhesion in primary human endothelial cells, J. Cell Sci., 115, 1837-1846.
Galkin, I. I., Pletjushkina, O. Y., Zinovkin, R. A., Zakharova, V. V., Birjukov, I. S., Chernyak, B. V., and Popova, E. N. (2014) Mitochondria-targeted antioxidants prevent TNFα-induced endothelial cell damage, Biochemistry (Moscow), 79, 124-130, https://doi.org/10.1134/S0006297914020059.
Rochfort, K. D., Collins, L. E., McLoughlin, A., and Cummins, P. M. (2016) Tumour necrosis factor-α-mediated disruption of cerebrovascular endothelial barrier integrity in vitro involves the production of proinflammatory interleukin-6, J. Neurochem., 136, 564-572, https://doi.org/10.1111/jnc.13408.
Pearlstein, D. P., Ali, M. H., Mungai, P. T., Hynes, K. L., Gewertz, B. L., and Schumacker, P. T. (2002) Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia, Arterioscler. Thromb. Vasc. Biol., 22, 566-573, https://doi.org/10.1161/01.atv.0000012262.76205.6a.
Lee, Y. W., Lee, W. H., and Kim, P. H. (2010) Oxidative mechanisms of IL-4-induced IL-6 expression in vascular endothelium, Cytokine, 49, 73-79, https://doi.org/10.1016/j.cyto.2009.08.009.
Murakami, M., Kamimura, D., and Hirano, T. (2019) Pleiotropy and specificity: insights from the interleukin 6 family of cytokines, Immunity, 50, 812-831, https://doi.org/10.1016/j.immuni.2019.03.027.
Rose-John, S. (2012) IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6, Int. J. Biol. Sci., 8, 1237-1247, https://doi.org/10.7150/ijbs.4989.
Valle, M. L., Dworshak, J., Sharma, A., Ibrahim, A. S., Al-Shabrawey, M., and Sharma, S. (2019) Inhibition of interleukin-6 trans-signaling prevents inflammation and endothelial barrier disruption in retinal endothelial cells, Exp. Eye Res., 178, 27-36, https://doi.org/10.1016/j.exer.2018.09.009.
Ali, M. I., Chen, X., and Didion, S. P. (2015) Heterozygous eNOS deficiency is associated with oxidative stress and endothelial dysfunction in diet-induced obesity, Physiol. Rep., 3, e12630, https://doi.org/10.14814/phy2.12630.
Schrader, L. I., Kinzenbaw, D. A., Johnson, A. W., Faraci, F. M., and Didion, S. P. (2007) IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy, Arterioscler. Thromb. Vasc. Biol., 27, 2576-2581, https://doi.org/10.1161/ATVBAHA.107.153080.
Wung, B. S., Ni, C. W., and Wang, D. L. (2005) ICAM-1 induction by TNFalpha and IL-6 is mediated by distinct pathways via Rac in endothelial cells, J. Biomed. Sci., 12, 91-101, https://doi.org/10.1007/s11373-004-8170-z.
Kaplanski, G., Marin, V., Montero-Julian, F., Mantovani, A., and Farnarier, C. (2003) IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation, Trends Immunol., 24, 25-29, https://doi.org/10.1016/s1471-4906(02)00013-3.
Ali, M. H., Schlidt, S. A., Chandel, N. S., Hynes, K. L., Schumacker, P. T., and Gewertz, B. L. (1999) Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction, Am. J. Physiol., 277, 1057-1065, https://doi.org/10.1152/ajplung.1999.277.5.L1057.
Alsaffar, H., Martino, N., Garrett, J. P., and Adam, A. P. (2018) Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis, Am. J. Physiol. Cell Physiol., 314, C589-C602, https://doi.org/10.1152/ajpcell.00235.2017.
Birukova, A. A., Tian, Y., Meliton, A., Leff, A., Wu, T., and Birukov, K. G. (2012) Stimulation of Rho signaling by pathologic mechanical stretch is a “second hit” to Rho-independent lung injury induced by IL-6, Am. J. Physiol. Lung Cell. Mol. Physiol., 302, L965-L975, https://doi.org/10.1152/ajplung.00292.2011.
Saura, M., Zaragoza, C., Bao, C., Herranz, B., Rodriguez-Puyol, M., and Lowenstein, C. J. (2006) Stat3 mediates interleukin-6 [correction of interelukin-6] inhibition of human endothelial nitric-oxide synthase expression, J. Biol. Chem., 281, 30057-30062, https://doi.org/10.1074/jbc.M606279200.
Hung, M.-J., Cherng, W.-J., Hung, M.-Y., Wu, H.-T., and Pang, J.-H. S. (2010) Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells, J. Hypertens., 28, 940-951, https://doi.org/10.1097/HJH.0b013e32833992ef.
Cohen, T., Nahari, D., Cerem, L. W., Neufeld, G., and Levi, B. Z. (1996) Interleukin 6 induces the expression of vascular endothelial growth factor, J. Biol. Chem., 271, 736-741, https://doi.org/10.1074/jbc.271.2.736.
Alagappan, V. K. T., Willems-Widyastuti, A., Seynhaeve, A. L. B., Garrelds, I. M., ten Hagen, T. L. M., Saxena, P. R., and Sharma, H. S. (2007) Vasoactive peptides upregulate mRNA expression and secretion of vascular endothelial growth factor in human airway smooth muscle cells, Cell Biochem. Biophys., 47, 109-118, https://doi.org/10.1385/cbb:47:1:109.
Murohara, T., Horowitz, J. R., Silver, M., Tsurumi, Y., Chen, D., Sullivan, A., and Isner, J. M. (1998) Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin, Circulation, 97, 99-107, https://doi.org/10.1161/01.cir.97.1.99.
Woodfin, A., Voisin, M.-B., and Nourshargh, S. (2007) PECAM-1: a multi-functional molecule in inflammation and vascular biology, Arterioscler. Thromb. Vasc. Biol., 27, 2514-2523, https://doi.org/10.1161/ATVBAHA.107.151456.
Zhang, J., Silva, T., Yarovinsky, T., Manes, T. D., Tavakoli, S., et al. (2010) VEGF blockade inhibits lymphocyte recruitment and ameliorates immune-mediated vascular remodeling, Circ. Res., 107, 408-417, https://doi.org/10.1161/CIRCRESAHA.109.210963.
Simmons, S., Erfinanda, L., Bartz, C., and Kuebler, W. M. (2019) Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation, J. Physiol., 597, 997-1021, https://doi.org/10.1113/JP276245.
Ke, Y., Oskolkova, O. V., Sarich, N., Tian, Y., Sitikov, A., et al. (2017) Effects of prostaglandin lipid mediators on agonist-induced lung endothelial permeability and inflammation, Am. J. Physiol. Lung Cell. Mol. Physiol., 313, 710-721, https://doi.org/10.1152/ajplung.00519.2016.
Tedgui, A., and Mallat, Z. (2006) Cytokines in atherosclerosis: pathogenic and regulatory pathways, Physiol. Rev., 86, 515-581, https://doi.org/10.1152/physrev.00024.2005.
Okajima, K. (2004) Prevention of endothelial cell injury by activated protein C: the molecular mechanism(s) and therapeutic implications, Curr. Vasc. Pharmacol., 2, 125-133, https://doi.org/10.2174/1570161043476429.
Ackermann, M., Verleden, S. E., Kuehnel, M., Haverich, A., Welte, T., et al. (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19, N. Engl. J. Med., 383, 120-128, https://doi.org/10.1056/NEJMoa2015432.
De Spiegelaere, W., Casteleyn, C., Van den Broeck, W., Plendl, J., Bahramsoltani, M., Simoens, P., Djonov, V., and Cornillie, P. (2012) Intussusceptive angiogenesis: a biologically relevant form of angiogenesis, J. Vasc. Res., 49, 390-404, https://doi.org/10.1159/000338278.
Armstrong, S. M., Mubareka, S., and Lee, W. L. (2013) The lung microvascular endothelium as a therapeutic target in severe influenza, Antiviral. Res., 99, 113-118, https://doi.org/10.1016/j.antiviral.2013.05.003.
Li, X., Molina-Molina, M., Abdul-Hafez, A., Ramirez, J., Serrano-Mollar, A., Xaubet, A., and Uhal, B. D. (2006) Extravascular sources of lung angiotensin peptide synthesis in idiopathic pulmonary fibrosis, Am. J. Physiol. Lung Cell. Mol. Physiol., 291, L887-L895, https://doi.org/10.1152/ajplung.00432.2005.
Liu, Y., Yang, Y., Zhang, C., Huang, F., Wang, F., et al. (2020) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury, Sci. China Life Sci., 63, 364-374, https://doi.org/10.1007/s11427-020-1643-8.
Forrester, S. J., Booz, G. W., Sigmund, C. D., Coffman, T. M., Kawai, T., et al. (2018) Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology, Physiol. Rev., 98, 1627-1738, https://doi.org/10.1152/physrev.00038.2017.
Griendling, K. K., Minieri, C. A., Ollerenshaw, J. D., and Alexander, R. W. (1994) Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells, Circ. Res., 74, 1141-1148, https://doi.org/10.1161/01.res.74.6.1141.
Nazarewicz, R. R., Dikalova, A. E., Bikineyeva, A., and Dikalov, S. I. (2013) Nox2 as a potential target of mitochondrial superoxide and its role in endothelial oxidative stress, Am. J. Physiol. Heart Circ. Physiol., 305, H1131-H1140, https://doi.org/10.1152/ajpheart.00063.2013.
Dikalov, S. I., and Ungvari, Z. (2013) Role of mitochondrial oxidative stress in hypertension, Am. J. Physiol. Heart Circ. Physiol., 305, H1417-H1427, https://doi.org/10.1152/ajpheart.00089.2013.
Itani, H. A., Dikalova, A. E., McMaster, W. G., Nazarewicz, R. R., Bikineyeva, A. T., Harrison, D. G., and Dikalov, S. I. (2016) Mitochondrial cyclophilin D in vascular oxidative stress and hypertension, Hypertension, 67, 1218-1227, https://doi.org/10.1161/HYPERTENSIONAHA.115.07085.
Bernardi, P., Rasola, A., Forte, M., and Lippe, G. (2015) The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology, Physiol. Rev., 95, 1111-1155, https://doi.org/10.1152/physrev.00001.2015.
Wassmann, S., Stumpf, M., Strehlow, K., Schmid, A., Schieffer, B., Böhm, M., and Nickenig, G. (2004) Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor, Circ. Res., 94, 534-541, https://doi.org/10.1161/01.RES.0000115557.25127.8D.
Imai, Y., Kuba, K., Rao, S., Huan, Y., Guo, F., et al. (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure, Nature, 436, 112-116, https://doi.org/10.1038/nature03712.
Kostapanos, M. S., Florentin, M., Elisaf, M. S., and Mikhailidis, D. P. (2013) Hemostatic factors and the metabolic syndrome, Curr. Vasc. Pharmacol., 11, 880-905, https://doi.org/10.2174/15701611113116660171.
Labinjoh, C., Newby, D. E., Dawson, P., Johnston, N. R., Ludlam, C. A., Boon, N. A., and Webb, D. J. (2000) Fibrinolytic actions of intra-arterial angiotensin II and bradykinin in vivo in man, Cardiovasc. Res., 47, 707-714, https://doi.org/10.1016/s0008-6363(00)00126-7.
Manne, B. K., Denorme, F., Middleton, E. A., Portier, I., Rowley, J. W., et al. (2020) Platelet gene expression and function in COVID-19 patients, Blood, 136, 1317-1329, https://doi.org/10.1182/blood.2020007214.
Aumiller, V., Balsara, N., Wilhelm, J., Günther, A., and Königshoff, M. (2013) WNT/β-catenin signaling induces IL-1β expression by alveolar epithelial cells in pulmonary fibrosis, Am. J. Respir. Cell. Mol. Biol., 49, 96-104, https://doi.org/10.1165/rcmb.2012-0524OC.
Deng, J., Wang, D.-X., Deng, W., Li, C.-Y., and Tong, J. (2012) The effect of endogenous angiotensin II on alveolar fluid clearance in rats with acute lung injury, Can. Respir. J., 19, 311-318, https://doi.org/10.1155/2012/951025.
Buckley, S. T., Medina, C., and Ehrhardt, C. (2010) Differential susceptibility to epithelial-mesenchymal transition (EMT) of alveolar, bronchial and intestinal epithelial cells in vitro and the effect of angiotensin II receptor inhibition, Cell Tissue Res., 342, 39-51, https://doi.org/10.1007/s00441-010-1029-x.
Wang, R., Zagariya, A., Ibarra-Sunga, O., Gidea, C., Ang, E., et al. (1999) Angiotensin II induces apoptosis in human and rat alveolar epithelial cells, Am. J. Physiol., 276, 885-889, https://doi.org/10.1152/ajplung.1999.276.5.L885.
Sriram, K., and Insel, P. A. (2020) A hypothesis for pathobiology and treatment of COVID-19: the centrality of ACE1/ACE2 imbalance, Br. J. Pharmacol., 177, 4825-4844, https://doi.org/10.1111/bph.15082.
Reynolds, H. R., Adhikari, S., Pulgarin, C., Troxel, A. B., Iturrate, E., et al. (2020) Renin-angiotensin-aldosterone system inhibitors and Rrsk of Covid-19, N. Engl. J. Med., 382, 2441-2448, https://doi.org/10.1056/NEJMoa2008975.
Monteil, V., Kwon, H., Prado, P., Hagelkrüys, A., Wimmer, R. A., et al. (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell, 181, 905-913.e7, https://doi.org/10.1016/j.cell.2020.04.004.
Hamming, I., Timens, W., Bulthuis, M. L. C., Lely, A. T., Navis, G. J., and van Goor, H. (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis, J. Pathol., 203, 631-637, https://doi.org/10.1002/path.1570.
Huang, R.-T., Wu, D., Meliton, A., Oh, M.-J., Krause, M., et al. (2017) Experimental lung injury reduces Krüppel-like factor 2 to increase endothelial permeability via regulation of RAPGEF3-Rac1 signaling, Am. J. Respir. Crit. Care Med., 195, 639-651, https://doi.org/10.1164/rccm.201604-0668OC.
Jha, P., and Das, H. (2017) KLF2 in regulation of NF-κB-mediated immune cell function and inflammation, Int. J. Mol. Sci., 18, 2383, https://doi.org/10.3390/ijms18112383.
Fledderus, J. O., Boon, R. A., Volger, O. L., Hurttila, H., Ylä-Herttuala, S., et al. (2008) KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells, Arterioscler. Thromb. Vasc. Biol., 28, 1339-1346, https://doi.org/10.1161/ATVBAHA.108.165811.
Ibrahim, H., Perl, A., Smith, D., Lewis, T., Kon, Z., et al. (2020) Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous n-acetylcysteine, Clin. Immunol., 219, 108544, https://doi.org/10.1016/j.clim.2020.108544.
Assimakopoulos, S. F., and Marangos, M. (2020) N-acetyl-cysteine may prevent COVID-19-associated cytokine storm and acute respiratory distress syndrome, Med. Hypotheses, 140, 109778, https://doi.org/10.1016/j.mehy.2020.109778.
Poe, F. L., and Corn, J. (2020) N-Acetylcysteine: A potential therapeutic agent for SARS-CoV-2, Med. Hypotheses, 143, 109862, https://doi.org/10.1016/j.mehy.2020.109862.
To, E. E., Erlich, J. R., Liong, F., Luong, R., Liong, S., et al. (2020) Mitochondrial reactive oxygen species contribute to pathological inflammation during influenza A virus infection in mice, Antioxid. Redox Signal., 32, 929-942, https://doi.org/10.1089/ars.2019.7727.
Winn, R. K., and Harlan, J. M. (2005) The role of endothelial cell apoptosis in inflammatory and immune diseases, J. Thromb. Haemost., 3, 1815-1824, https://doi.org/10.1111/j.1538-7836.2005.01378.x.
Wang, C., Luo, Z., Carter, G., Wellstein, A., Jose, P. A., et al. (2018) NRF2 prevents hypertension, increased ADMA, microvascular oxidative stress, and dysfunction in mice with two weeks of ANG II infusion, Am. J. Physiol. Regul. Integr. Comp. Physiol., 314, R399-R406, https://doi.org/10.1152/ajpregu.00122.2017.
Lopes, R. A., Neves, K. B., Tostes, R. C., Montezano, A. C., and Touyz, R. M. (2015) Downregulation of nuclear factor erythroid 2-related factor and associated antioxidant genes contributes to redox-sensitive vascular dysfunction in hypertension, Hypertension, 66, 1240-1250, https://doi.org/10.1161/HYPERTENSIONAHA.115.06163.
Zinovkin, R. A., and Grebenchikov, O. A. (2020) Transcription factor Nrf2 as a potential therapeutic target for prevention of cytokine storm in COVID-19 patients, Biochemistry (Moscow), 85, 978-983, https://doi.org/10.1134/S0006297920070111.
Acknowledgements
The authors express their gratitude to Professor, Academician of the Russian Academy of Sciences Vladimir Petrovich Skulachev, without whom this work would not come into being.
Funding
This study was financially supported by the Russian Foundation for Basic Research (projects nos. 20-04-60452 and 17-00-00088).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Chernyak, B.V., Popova, E.N., Prikhodko, A.S. et al. COVID-19 and Oxidative Stress. Biochemistry Moscow 85, 1543–1553 (2020). https://doi.org/10.1134/S0006297920120068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920120068