Abstract
Recently there has been a more focus on the development of an efficient technique for detection of circulating tumor cells (CTCs), due to their significance in prognosis and therapy of metastatic cancer. However, it remains a challenge because of the low count of CTCs in the blood. Herein, a rapid and high-sensitivity approach for CTCs detection using an integrated microfluidic system, consisting of a deterministic lateral displacement (DLD) isolating structure, an automatic purifying device with CD45-labeled immunomagnetic beads and a capturing platform coated with rat-tail collagen was reported. We observed high capture rate of 90%, purity of about 50% and viability of more than 90% at the high throughput of 1 mL/min by capturing green fluorescent protein (GFP)-positive cells from blood. Further capturing of CTCs from metastatic cancers patients revealed a positive capture rate of 83.3%. Furthermore, our device was compared with CellSearch system via parallel analysis of 30 cancer patients, to find no significant difference between the capture efficiency of both methods. However, our device displayed advantage in terms of time, sample volume and cost for analysis. Thus, our integrated device with sterile environment and convenient use will be a promising platform for CTCs detection with potential clinical application.
Similar content being viewed by others
Introduction
Cancer has become a major public health problem, due to its association with most number of patient deaths worldwide1. Primarily, it involved tumor cell metastasis2,3, wherein tumor cells escape the primary lesions, and penetrate into lymphatic or blood vessel system, through which they migrate comfortably to distant places and form metastatic colonies4. Circulating tumor cells (CTCs) are defined as tumor cells typically present in the body circulating system. They have generally been considered as “liquid biopsies”, which can be used as a minimally invasive method for diagnosis and assessing cancer status, estimating prognosis, evaluating efficacy and instructing personal therapy5,6,7. However, there has been a tremendous challenge in detecting and capturing these CTCs due to their extreme rarity, with the presence of only 1 to 100 CTCs in 109 blood cells8.
Apparently, in the past decade, numerous methods have been developed to isolate the CTCs population based on their properties, particularly their physical or biological properties that differentiate them from blood cells. These mainly include size filtration, density gradient and di-electrophoresis9,10,11,12,13, which are label-free, convenient and low cost, but have some limitations. For instance, in size filtration method, the clogging due to other blood cell types, makes CTCs capture difficult14. Recently the immune-based CTCs separation method involving the heterogeneous expression of surface markers, such as epithelial cell adhesion molecule (EpCAM) has been shown to be useful15. The CellSearch system is the only commercial detection system approved by the US Food and Drug Administration (FDA), which uses ferro-fluids conjugated with anti-EpCAM antibody, to magnetically enrich CTCs16, and has already been used in the clinic to monitor the response of patients with breast, prostate, and colorectal cancers to specific treatments. Nevertheless, a multi-institutional study involving 177 patients with measurable metastatic breast cancer, reported that CTCs were detected in only 61% of the patients by this system17. This has mainly been attributed to the heterogeneous expression of EpCAM18,19,20,21,22,23,24. Despite the presence of many potential approaches to isolate and purify CTCs, there several issues must be addressed to realize the full potential of CTCs as a diagnostic and research tool.
Thus, to overcome the issues, microfluidic technology, which literally represents lab on a chip, and has the advantages of high throughput, integration, adjustment and management, with low cost and small volume, may solve the problems. Until now, various CTCs detection methods using microfluidic system have been analyzed, with different cell separation mechanisms, such as size or deformability based isolation, dielectrophoresis, affinity chromatography and magnetic forces25,26,27,28. These physical properties based separation systems offer the advantages of label-free sorting, high throughput, and low cost29,30. However, these methods often results in poor capture rate and poor purity by ignoring the interference of WBCs, and CTCs are also susceptible to damage from large mechanical stresses27. Moreover, the immune-based methods to detect CTCs also display few limitations. For instance, it costs too much time for CTCs to react with the antibodies coated on the chip due to the interference and hindrance by other blood components. Subsequently, these factors result in low capture efficiency. Thus, if blood samples could somehow be processed early, then affinity based capturing of CTCs can yield better results.
Hence, our group has developed an integrated microfluidic system that has the potential to overcome the obstacles of any individual method. In the past, we developed an integrated microfluidic system, that included a DLD and a fishbone structure10. However, to avoid clogging, blood had to be diluted before adding to the microchannels of the DLD structure. And some WBCs might get stuck in the corners of the fishbone structure. In this study, we have tried to improve the DLD structure, where blood samples needn’t to be diluted to have more convenient and rapid detection. In addition, we have also tried to develop an automatic magnetic purifying device for negative isolation, which decrease the false positive rate to a greater extent, and enhance CTCs purity. In fact with our developed method, CTCs will be captured by the sequential elimination of blood cells in the novel method, where nearly all RBCs and platelets and more than 90% of the WBCs will be first depleted by DLD isolating chip. The remaining small percentage of WBCs will be purified and removed by the automatic immunomagnetic purifying device. Finally, CTCs will be captured on a platform coated with rat-tail collagen, where CTCs are distinguished based on the immune affinity31. The integrated system has demonstrated its usefulness not only in artificially spiked blood samples, but also in clinical samples obtained from various advance cancer patients. We observed a capture rate of around 90%, capture purity of about 50% at the high throughput of 1 mL/min, and the viability of CTCs detected through this method reached more than 90%. Furthermore capturing of CTCs using the novel device specifically from the peripheral blood of patients with various advanced metastatic cancers revealed a positive capture rate of 83.3%. In addition, the efficiency of the novel device was compared with CellSearch system, and we observed equal and excellent capture efficiency just as it. What’s more, the results of CTCs detection were in accordance with the clinical condition. Nevertheless, the novel device displayed the advantages in terms of less time, volume and cost required for the analysis. Finally, it is an integrated system which is automatic, sterile and has the convenience of manipulation for universal adaptation, based on the requirement.
Results
Design and functioning of the combined microfluidic chip
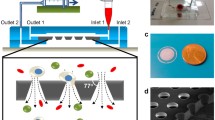
The newly developed DLD chip was shown in (Fig. 1b). We introduced one chamber with dimensions of 68 mm long, 3.5 mm wide and 50 μm high, two inlets, and two outlets, which was instead of the 8 parallel chambers with dimensions of 42 mm long, 3.5 mm wide and 30 μm high, only one inlet and outlet in the previous design. Moreover, the size of the microarray was also optimized. The new microarray consisted of microposts with 25 μm radius, 30 μm horizontal gaps and 20 μm vertical gaps, with a tilted angle of 3.2 degree towards the fluid flow direction. The cell separation mechanism in DLD array was based on the interaction of cells with microposts under low Reynolds number conditions. As shown in (Fig. 1d,e), microposts can divide the flow of fluid into several narrow streams, and when cells pass through the gaps in microposts, smaller cells with radius less than the width of first stream L, are able to continue following the stream. On the contrary, large cells with radius larger than the width of first stream L, are forced to follow the second stream with a deterministic path. Based on this analysis, 2L can be regarded as the critical cells size. According to the theory proposed by Loutherback et al.32, the critical separation size of our DLD structure was 6.75 μm. Compared with the previous design, the optimization of the new structure led us to isolate blood samples without diluting with a high throughput of about 1 mL/min, and 3 mL blood would be detected within 3 min. After the first isolation, CTCs with the residual WBCs were collected and subjected to further purification.
(a) Schematic illustration of the integrated device included isolating chip and the purifying device. The purifying device consisted of purifying chip, the permanent magnet and the step** motor. Blood sample was pumped into the isolating chip for a preliminary isolation and then passed on to the purifying chip, where CD45 dynabeads were added, and WBCs were eventually removed by the magnet. (b) Schematic illustration of the DLD chip. The microfluidic deterministic lateral displacement (DLD) chamber consisted of mirrored triangular micropost array. The large cells (cancer cells and part of leukocytes) from the blood would concentrate in the center of chamber, while small cells (erythrocytes and most of leukocytes) will follow the streamline direction. (c) Schematic illustration of the cancer cell purifying chip which consisted of three layers, including a layer for inlet and outlet, a layer for the groove for purified cells and a layer for the base. (d,e) Schematic illustration of cell separation in DLD micropost array. The fluid (blood sample) flowing through the DLD structures was partitioned into different streams, in which large cells (including cancer cells and some of the leukocytes) flowed in a bum** mode and thus concentrated in the center of the chamber, while small cells (erythrocytes, platelets and most of the leukocytes) followed the direction of the flow.
Next, a negative isolation based automatic magnetic purifying device was developed instead of the fishbone chip labeled with anti-EpCAM antibody. The size of chip was based on the volume of the samples, and as 200 μL fluid was collected from DLD from 1 mL of blood volume, we applied 600 mm3 for patients blood samples purifying. The chip chamber was fabricated with a 20 (Length) × 10 (Width) × 3 (Height) mm3 size, and a corresponding computer code was written to move the magnet within the borders of the chamber. The movement of the magnet was in a rectangular shape, as it moved from one length to another and one width to another, with a round trip.
In addition, the best parameters were also standardized, including the binding rate (the number of WBCs binding to CD45 dynabeads/all the WBCs) and the binding time. The human WBCs were isolated from the healthy volunteers by using RBC Lysis Buffer (1:10, eBioscience) and CD45 immunomagnetic dynabeads (Invitrogen, USA). As the recommended ratio and time were 5:1 (Dynabeads/WBCs) at 30 min, different ratios (5:1, 10:1, 15:1) and different time (10 min, 20 min, 30 min) points were tried. The binding time of 20 min displayed the binding rate of 40.09% (5:1), 82.95% (10:1) and 85.25% (15:1), (Fig. 2a). However at a ratio of 10:1, the binding rate was 36.9% (10 min), 82.95% (20 min) and 85.6% (30 min), (Fig. 2b) Finally, the 10:1 ratio and 20 min time were identified as best parameters, and under this condition, WBCs were binding to the dynabeads sufficiently (Fig. 2c). Further, to verify the advantage of the novel developed device, the similar experiments was performed with the traditional method using DH-II Rotary mixing apparatus (** them onto the DLD chip with OB1 Pressure Controller (ELVEFLOW, France). This pressure controller had the advantage over the traditional injection pump, in terms of maintaining uniform force to move the fluid more stably, and also simultaneously providing different forces through different passageways.
First, under pressure exerted by a OB1 Pressure Controller (ELVEFLOW, France), the blood specimens were pumped into the Inlet A2, and PBS was injected into the Inlet A1 to balance the pressure. The section (waste) containing the RBCs and most WBCs was collected at Outlet B2, while CTCs and the residual WBCs passed through Outlet B1, which was subsequently connected to the inlet of the automatic purifying device by a flexible plastic tube (TYGON, USA). CD45 Dynabeads were then added into the purifying chip through the outlet. To improve the interaction between anti-CD-45 Dynabeads and WBCs, we developed the purifying device, in which Dynabeads were forced via magnet to move around the chamber to enhance their interaction with WBCs. Further, to ensure that captured WBCs and Dynabeads were removed, the magnet from the purifying device was allowed to rest at the bottom of the chamber, and the supernatant containing CTCs and uncaptured WBCs was sucked out. Finally, cells were captured on the rat-tail coated dish and counted under microscope. The capture rate and capture purity were calculated with the following equation: Capture rate = C1/N %; Capture purity = C1/C2%; where C1 is the number of cancer cells captured from the chip, and N is the number of all the cancer cells pumped at the starting point, C2 is the number of all the cells (cancer cells and white blood cells) captured from the device.
Next, the viability of the captured cells was measured by a standard Live/Dead fluorescent assay (Hochest/ PI staining), whose result was compared with the control cells that were never introduced into the microfluidic device. Both these cell types were cultured at 37 °C with 5% CO2 and the medium was changed every 1–2 days, and the growth and proliferation were observed every day.
CTCs isolation and capture from metastatic cancer patients
Furthermore, the blood specimen (3 mL) of patients with advanced metastatic cancer, who were recruited at The Second Hospital Of Dalian Medical University, PR China, were collected for CTCs detection after their informed consent. The experimental protocol was approved by the Ethics Review Committee of the Second Hospital of Dalian Medical University, and this study was conducted in compliance with ethical and safe research practices involving human subjects or blood. Clinical characteristics and information of the patients were obtained from their medical records. These blood specimens were also collected into ethylene diamine tetraacetic (EDTA)-containing tubes and processed within 6 hours as outlined in the above paragraph. The clinical history of each patient was also considered, which contributed to the analysis of the results of CTCs detection by the novel device.
Comparison of the novel device with CellSearch System for capture efficiency
To further test the novel device, more blood samples from advanced metastatic cancer patients, were collected. Each patient donated 10.5 mL of blood for detection, wherein 7.5 mL was processed with Cell Search System, while 3 mL volume was analyzed in the novel device. Samples detected by CellSearch were processed within 96 hrs. Capture efficiency by the two methods were compared by the peripheral blood samples from 30 patients with advanced metastatic cancer. The condition of the each patient, including gender, age, diagnosis and therapy was also recorded, to correlate the results of CTCs detection by the two methods.
Statistical analysis
All statistical analyses were carried out using SPSS 13.0 statistical software package (Chicago, IL, USA). Data were expressed as mean ± standard deviation (SD). Statistical significance of the differences between experimental groups was determined using Student’s t-test. P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Jiang, J. et al. An integrated microfluidic device for rapid and high-sensitivity analysis of circulating tumor cells. Sci. Rep. 7, 42612; doi: 10.1038/srep42612 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics 2016. CA: A Cancer Journal for Clinicans 66, 7–30 (2016).
Zhao, H. et al. CD47 Promotes Tumor Invasion and Metastasis in Non-small Cell Lung Cancer. Scientific Reports 6, 29719 (2016).
Wang, C. et al. Simultaneous isolation and detection of circulating tumor cells with a microfluidic silicon-nanowire-array integrated with magnetic up conversion nanoprobes. Biomaterials 54, 55–62 (2016).
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331, 1559–64 (2016).
Harb, W. et al. Mutational analysis of circulating tumor cells using a novel microfluidic collection device and qPCR assay. Transl Oncol 6, 528–38 (2013).
Attard, G. et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res 69, 2912–8 (2009).
Chiu, T. K. et al. Application of optically-induced-dielectrophoresis in microfluidic system for purification of circulating tumour cells for gene expression analysis-Cancer cell line model. Scientific Reports 6, 32851(2016).
Yap, T. A., Lorente, D., Omlin, A., Olmos, D. & de Bono, J. S. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res 20, 2553–68 (2014).
Ciam, I. et al. Label free isolation of circulating tumor cells in microfluidic devices: Current research and perspectives. Biomicrofluidics 7, 11810 (2013).
Liu, Z. et al. High throughput capture of circulating tumor cells using an integrated microfluidic system. Biosensors and Bioelectronics 47, 113–9 (2013).
Xu, H. et al. Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials 32, 9758–65 (2011).
Fan, X. et al. A microfluidic chip integrated with a high-density PDMS-based mi- crofiltration membrane for rapid isolation and detection of circulating tumor cells. Biosensors and Bioelectronics 71, 380–6 (2015).
Alix-Panabières, C. & Pantel, K. Challenges in circulating tumor cell research. Nature Reviews Cancer 14, 623–31(2014).
Gach, P. C. et al. Micropallet arrays for the capture, isolation and culture of circulating tumor cells from whole blood of mice engrafted with primary human pancreatic adenocarcinoma. Biosensors and Bioelectronics 54, 476–83 (2014).
Shao, J. et al. Warfarin and coumarin-like Murraya paniculata extract down-regulate EpCAM-mediated cell adhesion: individual components versus mixture for studying botanical metastatic chemopreventives. Scientific Reports 6, 30549 (2016).
Olmos, D. et al. Circulating tumor cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann Oncol 20, 27–33 (2009).
Allard, W. J. et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10, 6897–904 (2004).
Frank, A. W. et al. Challenges in the Enumeration and Phenoty** of CTC. Clin Cancer Res 20, 7511–8 (2012).
de Albuquerque, A., Kaul, S., Breier, G., Krabisch, P. & Fersis, N. Multimarker Analysis of Circulating Tumor Cells in Peripheral Blood of Metastatic Breast Cancer Patients: A Step Forward in Personalized Medicine. Breast Care 7, 7–12 (2012).
Matthew, G. et al. Molecular analysis of circulating tumor cells —biology and biomarkers. Nature Reviews Clinical Oncology 11, 129–44 (2014).
Guo, S. et al. Degradable Zinc-Phosphate-Based Hierarchical Nanosubstrates for Capture and Release of Circulating Tumor Cells. ACS Appl. Mater. Interfaces 25, 15917–25 (2016).
Zeisberg, M. & Neilson, E. G. Biomarkers for epithelial-mesenchymal transitions. The Journal of Clinical Investigation 119, 1429–37 (2009).
Lu, H. et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nature Cell Biology 16, 1105–30 (2014).
Aceto, N. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–22 (2014).
Nagrath, S. et al. Isolation of rare circulating tumor cells in cancer patients by microchip technology. Nature 450, 1235–9 (2007).
Sequist, L. V. et al. An Exciting New Tool to Detect Circulating Tumor Cells in Lung Cancer Patients. Journal of Thoracic Oncology 4, 281–3 (2009).
Li, P. et al. Probing circulating tumor cells in microfluidics. Lab Chip 4, 602–9 (2013).
Adams, D. L., Alpaugh, R. K., Tsai, S., Tang, C. M. & Stefansson, S. Multi-Phenotypic subty** of circulating tumor cells using sequential fluorescent quenching and restaining. Scientific Reports 6, 33488 (2016).
Mao, X. & Huang, T. J. Exploiting mechanical biomarkers in microfluidics. Lab Chip 12, 4006–9 (2012).
Choi, H. et al. A label-free DC impedance-based microcytometer for circulating rare cancer cell counting. Lab Chip 13, 970 (2013).
Zhang, L., Wang, J., Zhao, L., Meng, Q. & Wang, Q. Analysis of chemoresistance in lung cancer with a simple microfluidic device. Electrophoresis 31, 3763–70 (2010).
Loutherback, K. et al. Improved performance of deterministic lateral displacement arrays with triangular posts. Microfluid Nanofluid 9, 1143–9 (2010).
Yeo, T. et al. Microfluidic enrichment for the single cell analysis of circulating tumor cells. Scientific Reports 6, 22076 (2016).
Vishnoi, M. et al. The isolation and characterization of CTC subsets related to breast cancer dormancy. Scientific reports 5, 27533 (2015).
Alix-Panabieres, C. & Pantel, K. The circulating tumor cells: liquid biopsy of cancer. Klin Lab. Diagn 4, 60–4 (2014).
Alix-Panabières, C. & Pantel, K. Challenges in circulating tumor cell research. Nat Rev Cancer 14, 623–31(2014).
Lu, N. et al. Biotin-Triggered Decomposable Immunomagnetic Beads for Capture and Release of Circulating Tumor Cells. ACS Appl. Mater. Interfaces 16, 8817–26 (2015).
Li, X., Chen, W., Liu, G., Lu, W. & Fu, J. Continuous-flow microfluidic blood cell sorting for unprocessed whole blood using surface micromachined microfiltration membranes. Lab on a chip 14, 2565–75(2014).
Loutherback, K. et al. Deterministic separation of cancer cells from blood at 10 mL/min. AIP Advances 2(4), 42107 (2012).
Gupta, V. et al. ApoStream(™), a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics 6(2), 24133 (2012).
Hyun, K. A., Lee, T. Y., Lee, S. H. & Jung, H. I. Two-stage microfluidic chip for selective isolation of circulating tumor cells (CTCs). Biosensors and Bioelectronics 67, 86–92 (2015).
Huang, Y. et al. Screening and Molecular Analysis of Single Circulating Tumor Cells Using Micromagnet Array. Scientific Reports 5, 16047 (2015).
Ozkumuret, E. et al. Inertial Focusing for Tumor Antigen–Dependent and –Independent Sorting of Rare CirculatingTumor Cells. Sci Transl Med 5, 179–89 (2015).
Andree, K. C. et al. Terstappen. Challenges in circulating tumor cell detection by the CellSearch system. Molecular oncology 10, 395–407 (2016).
David, T. et al. RNA-Seq of single prostate CTC simplicates noncanonical Wnt signaling in antiandrogen resistance. Science 349, 1351–7 (2016).
Neuert, G. et al. Systematic Identification of Signal-Activated Stochastic Gene Regulation. Science 339, 584–90 (2013).
Yu, M. et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 339, 580–4 (2013).
Xu, Z. et al. Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 34, 4109–17 (2013).
Grzybowski, B. A., Stone, H. A. & Whitesides, G. M. Dynamic self-assembly of magnetized, millimetre-sized objects rotating at a liquid-air interface. Nature 405, 1033–6 (2000).
Meng, Q. et al. Development of a double-layer microfluidic chip with flow medium for chemotherapy resistance analysis of lung cancer. Electrophoresis 32, 3446–53 (2011).
Acknowledgements
We express our gratitude to the all patients who participated in this study and the healthy volunteers who contributed blood samples. This work was supported in part by grants from the National High Technology Research and Development Program (863 Program Projects) of China (#2015AA020409), the National Natural Science Foundation of China (#91129733, #81330060 and #81502702), and Technology Plan Foundation of Liaoning Province (2014225003).
Author information
Authors and Affiliations
Contributions
J.J., H.Z., W.S. and J.T. performed the experiments. J.J. and Q.W. designed the study. Y.H., Y.S., R.W. and E.L. provided technical help. J.J., D.S., D.H., and X.D. performed statistical analysis. J.J., Q.W., Y.C. and L.Z. wrote the manuscript. L.Z., Y.C. and Q.W. provided scientific discussion. All authors contributed to the discussion and review of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jiang, J., Zhao, H., Shu, W. et al. An integrated microfluidic device for rapid and high-sensitivity analysis of circulating tumor cells. Sci Rep 7, 42612 (2017). https://doi.org/10.1038/srep42612
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42612
- Springer Nature Limited
This article is cited by
-
Stiffness influence on particle separation in polydimethylsiloxane-based deterministic lateral displacement devices
Microfluidics and Nanofluidics (2023)
-
Increasing flow rates in polydimethylsiloxane-based deterministic lateral displacement devices for sub-micrometer particle separation
Microfluidics and Nanofluidics (2023)
-
Geometric structure design of passive label-free microfluidic systems for biological micro-object separation
Microsystems & Nanoengineering (2022)
-
Circulating tumor cells in colorectal cancer in the era of precision medicine
Journal of Molecular Medicine (2022)
-
Detection and clinical significance of circulating tumor cells in colorectal cancer
Biomarker Research (2021)