Abstract
Southern Asian flowers offer honeybees a diversity of nectar. Based on its geographical origin, honey quality varies. Traditional methods are less authentic than DNA-based identification. The origin of honey is determined by pollen, polyphenolic, and macro-microorganisms. In this study, amplicon sequencing targets macro-microorganisms in eDNA using the ITS1 region to explore honey’s geographical location and authentication. The variety of honey samples was investigated using ITS1 with Illumina sequencing. For all four honey samples, raw sequence reads showed 979,380 raw ITS1 amplicon reads and 375 ASVs up to the phylum level. The highest total number of 202 ASVs up to phylum level identified Bali honey with 211,189 reads, followed by Banggi honey with 309,207 a total number of 111 ASVs, and Lombok represents only 63 ASVs up to phylum level with several read 458,984. Based on Shannon and Chao1, honey samples from Bali (B2) and (B3) exhibited higher diversity than honey from Lombok (B1) and green honey from Sabah (B4), while the Simpson index showed that Banggi honey (B4) had higher diversity. Honey samples had significant variance in mycobiome taxonomic composition and abundance. Zygosaccharomyces and Aspergillus were the main genera found in Lombok honey, with percentages of 68.81% and 29.76% respectively. Bali honey samples (B2 and B3) were identified as having a significant amount of the genus Aureobasidium, accounting for 40.81% and 25% of the readings, respectively. The microbiome composition of Banggi honey (B4) showed a high presence of Zygosaccharomyces 45.17% and Aureobasidium 35.24%. The ITS1 analysis effectively distinguishes between honey samples of different origins and its potential as a discriminatory tool for honey origin and authentication purposes.
Similar content being viewed by others
Introduction
The floral source determines the honey’s color, flavor, and aroma, by the distinctions between light and mild clover honey and dark, robust buckwheat honey. Mineral content in nectar influences honey’s physical properties, including electrical conductivity. Furthermore, plant-derived secondary metabolites in nectar contribute to honey’s antioxidant and antibacterial qualities, imparting unique floral nuances1,2,3. Nectar, the primary resource for honey production, exhibits a complex composition crucial for sustaining bee colonies.
Comprising sugars in the range of 20–80%, with sucrose, fructose, and glucose serves as a concentrated energy source for bees. Additionally, nectar contains 70–80% water, requiring enzymatic processing by honeybees to reduce it for prolonged storage. Trace elements such as amino acids, proteins, vitamins, minerals, and secondary metabolites contribute to honey’s distinctive flavor, color, and antibacterial attributes. The search for honey of the highest caliber, free of any human contamination, and abundant in natural constituents is of utmost importance4. Honey, mainly for its high osmolarity, low water activity, natural acidity, and low protein content, does not favor the growth of microorganisms5.
The microbes acquired in honey are likely derived from many sources, including pollen, soil, dust, water, Meliponini’s digestive tract, air, and nectar, which are challenging to control. Furthermore, inadequate attention to hygiene during honey processing, handling, and storage can result in post-harvest microbial contamination6. Fungi are a prominent Kingdom within the Eukaryotic sphere of life. Certain species possess significant economic value as they produce antibiotics, aid in the fermentation of food products like beer and bread, and facilitate the breakdown of cellulose. The number of fungal species is expected to be in the millions, yet only a limited few have been thoroughly described7. Fungi that are frequently present in plants and soil can be transferred to beehives via the process of pollination. The plant-associated microbes can be found in honey and other bee products such as bee bread, which is a fermented mixture of pollen and nectar used as food for bees. Some of these microbes have a positive impact on the bee colonies8.
The mycobiome refers to the fungal part of the microbiome. The term was initially employed specifically about the human mycobiome7. Yeast was the dominant fungi in honey identified using culture and molecular-based methods, such as Zygosaccharomyces, Debaryomyces, and Candida, have been previously identified in honey9. The ubiquitous Aspergillus, functioning as an opportunistic pathogen, poses a threat to honeybee larvae and triggers stonebrood disease. Ascosphaera apis, the causative agent of chalkbrood disease, and spore-forming fungi Nosema apis and Nosema ceranae are contributors to nosema disease10,11. One of the most prevalent types of fungi identified in honey is Cladosporium, a filamentous fungus that is ubiquitous and, according to some assumptions, could even live in concord with bees, spread from plant to bee, and then settle into bee products8. Honey is often contaminated with filamentous fungi, such as Cladosporium, Alternaria, and Aspergillus9.
The chemical features of honey, such as its low water activity, low pH, and presence of hydrogen peroxide, prevent the growth of microorganisms. As a result, latent forms of microbes, such as bacterial spores and yeasts, are commonly noticed in honey. The particles will originate from the flowers that the bees visit, as well as from the immediate environment of the hive (such as the soil and water), and from the hive and the bees themselves. Therefore, they can potentially offer additional evidence regarding the sources of honey3.
Few studies have investigated fungal species on bees, however functional studies show that yeasts impact bee growth, pathogen interactions, and foraging. Most fungi are either facultative bees associated with uncertain or environmentally contextual effects; the rare ones are obligately beneficial symbionts12. Honey contains eDNA from both microbes and bees, offering valuable insights into the hive microbiome and the honeybee hologenome. Consequently, eDNA analysis sheds light on bee pathospheres and overall colony health13,14,15,16. To refine the source attribution, incorporating data from diverse taxonomic groups detected in honey alongside plant-derived information allows for the application of DNA-based techniques to comprehensively identify the honey’s microbial constituents2,3.
Analyzing the honey microbiome may illuminate beehive dynamics, bolster honey production, and combat honeybee diseases. However, prior studies predominantly employ culture-based methodologies, which may entail culture-driven biases17. Culture-independent techniques, exemplified by next-generation sequencing, have begun to explore honeybee-associated microbial realms, unveiling insights into the microbiome of honeybee gastrointestinal tracts, pollen, and bee bread, although metagenomic analyses of honey remain limited10,14,18,19,20.
The DNA traces provide evidence of the micro macro-organisms that the bees have come into physical contact with, whether it was intentional or unintentional. Many studies suggest that identification of plant, fungal and bacterial taxonomic groups play a role as in identification of honey origin and separating honey samples of different origin3. Thus, the purpose of this study to demonstrate the prospect of DNA-based approaches in determining a fungal taxonomic group in newly selected area. To thoroughly examine the presence of micro and macro-organisms (specifically the fungal community) in honey and to provide a comprehensive understanding of honey quality, authentication from various locations in Lombok, Bali and Banggi Island. A culture-independent method ITS1 was utilized to obtain a detailed description of the fungal microbiota present in the honey samples. The microbial fingerprints of honey, particularly its fungal diversity, hold immense potential for revealing its origin and floral sources.
This study aims to examine the ITS1 diversity (alpha and beta) of honey samples from distinct regions—Lombok, Bali and Banggi. Ultimately, this investigation aspires to translate its findings into practical recommendations for safeguarding the regional microbial diversity paving the way for sustainable and authentic honey production practices.
Results
Amplicon data sequencing summary
A total of 979,380 raw reads were obtained from 12 samples, with the average raw reads and filtered reads detailed in (Table 1). Variations in G + C content (%) were observed among the samples. Bali honey (B2 and B3) exhibited the highest G + C content, suggesting a relatively higher proportion of guanine and cytosine nucleotides in its genetic makeup. In contrast, Banggi honey (B4) and Lombok (B1) showed comparatively lower G + C content, indicating distinct genetic characteristics (Table 1). These findings highlight the diversity and variability present in the sequence data obtained from different honey samples, providing insights into their genetic composition and potential differences between them.
Table 1 also provides a summary of the sequencing data obtained from honey samples collected from four distinct locations. The table outlines the number of samples analyzed from each location, along with the corresponding counts of raw reads and filtered reads after eliminating unidentified sequences of ITS1 amplicon reads. The data reveals notable differences in the quantity of reads obtained per sample across the locations. Lombok honey samples (B1) exhibited the highest average number of filtered sequencings reads per sample, followed by Banggi honey samples (B4).
In contrast, Bali honey samples (B2) displayed the lowest average number of filtered sequencings reads per sample, with a significantly lower value compared to the other locations. Moreover, the data presents the number of amplicon sequence variants (ASVs) identified for each type of honey sample. Despite similar values across locations, Banggi honey samples showed the highest average number of ASVs per sample. However, it is important to note the relatively high standard deviations for all locations, indicating variability in the bacterial and fungal communities present in honey samples from these locations.
Alpha diversity (richness) analysis of honey samples
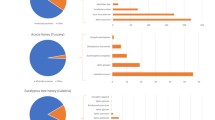
Table 2 provides a comprehensive overview of alpha diversity analysis conducted on honey samples collected from Lombok (B1), Bali (B2 and B3), and Banggi (B4). Alpha diversity, which denotes the variety of species within a single location, was assessed using multiple indices, including Chao1, Shannon, and Simpson. Chao1 Index specifies the Bali honey samples (B2) exhibited the highest estimated total number of species with a Chao1 value of 60.00 ± 31.11, followed by Bali (B3) (58.00 ± 19.79), Banggi (B4) (52.67 ± 16.36), and Lombok (B1) (35.62 ± 7.18). This suggests a potential gradient in species richness, with Bali (B2) harbouring the most diverse microbial community.
Shannon Index demonstrated Bali (B3) the highest evenness with a Shannon index value of 4.63 ± 1.19, followed by Bali (B2) (4.02 ± 1.88). B2 and B3 located in the same island. Lower values were observed in Banggi (B4) (1.87 ± 1.33) and Lombok (B1) (1.59 ± 0.058), indicating a less even distribution of species in these samples.
Simpson Index on Banggi honey (B4) displayed the highest diversity (0.39 ± 0.24), followed by Lombok (B1) (0.51 ± 0.038). Conversely, Bali honeys (B2 and B3) had lower values (0.78 ± 0.22 and 0.89 ± 0.092, respectively), indicating a higher dominance of specific microbial species in these samples. Combining the analysis of Chao1, Shannon, and Simpson indices, honey samples from Bali (B2) exhibit the highest overall alpha diversity in terms of species richness. However, Bali (B3) demonstrates a more balanced distribution of species within the community, as indicated by the Shannon index. Interestingly, Banggi honey (B4) displays the highest diversity based on the Simpson index, suggesting a lower dominance of specific microbial species. It is important to acknowledge the limitations of this analysis. Standard deviations associated with the diversity indices indicate variability within each location. Additionally, factors like sequencing depth and potential sampling biases might influence the observed diversity patterns.
Beta diversity analysis of honey samples
Beta diversity of honey samples from different sources was calculated using robust Aitchison, Principal Coordinate Analysis (PCoA), Bray–Curtis, Jaccard, weighted Unifrac, and unweighted Unifrac methods (Fig. 1). Beta diversity quantifies the variation in species composition among locations or habitats within a broader region. It complements alpha diversity, which refers to the variety within a single community.
Beta diversity represented by the rpca, Bray Curtis, Jaccard, weightated_unifarc and unweighted_unifarc. Each plot point represents a single sample and was colored and shaped by group according to the legend. Samples that clustered close together are more likely to share a similar microbial composition.
In our investigation, we noticed that honey samples forming tight clusters in the Principal Coordinate Analysis (PCoA) plot shared more similar mycobiome compositions, while those scattered further apart exhibited greater dissimilarities. Each location exhibited a distinct signature of genera and species, as evidenced by variations in Beta diversity metrics such as Bray–Curtis, Jaccard, weighted UniFrac, and unweighted UniFrac. These findings imply that environmental factors such as geography, flora, and fauna might influence the spatial diversity in honeybee foraging patterns and microbial composition. In the PCoA analysis, the proximity of points on the plot denotes similarities in fungal microbiota composition, while greater distances indicate more pronounced differences in fungal species types and quantities. The arrangement of individual samples on the plot reflects disparities in mycobiome composition, with the two principal components accounting for 91.56% of the variance, thereby representing the most significant dimensions of differentiation among the honey samples. Bali (B3) stands out prominently above Lombok (B1) and Bali (B2) due to its distinct fungal ecology, resulting in discernible plot segregation. Although the fungal community in Banggi (B4) closely resembles that of Bali (B3), its high-abundance taxa form a distinct cluster (Fig. 1).
The Bray–Curtis index, while primarily influenced by dominant taxa, is relatively less swayed by their dominance in honey samples. Approximately 74.98% of dissimilarities were elucidated by the two principal components analysis. In the plot, Lombok honey (B1) was positioned on the left side, while Banggi honey (B4) occupied the right side, indicating stark dissimilarities between these samples. Conversely, Bali (B3) and Banggi (B4) appeared relatively closer to each other in the middle of the plot, suggesting similarities in mycobiota and origin between these honey samples.
Regarding the Jaccard index, utilized for comparing the similarity between distinct groups, approximately 39.86% of similarities were explained by the first two principal components among all honey samples. A Jaccard index value close to 0 or negative suggests greater dissimilarities than similarities between samples from different groups, implying diverse fungal compositions among honey samples from different groups.
Weighted UniFrac is biased toward measuring abundant taxa in samples. Bali (B2) and Banggi (B4) honey samples exhibit greater diversity, with the highest value of 0.6 at PC2, followed by Bali (B3), and the lowest in Lombok (B1) honey. This suggests distinct fungal communities across all honey samples.
In contrast, the Unweighted UniFrac index is inclined towards rare taxa and is less sensitive to abundance variations. Based on this index, all honey samples appear more diverse and overlap with each other. The comprehensive analysis of beta diversity measurements through PCoA and various indices provides valuable insights into the unique microbial compositions of honey samples from different geographical regions. These differences likely arise from environmental factors influencing honeybee foraging behaviors and subsequent microbial populations in the honey.
Mycobiome community in honey
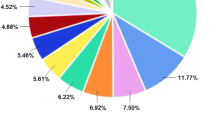
Figure 2 showing the overall taxonomy of microorganisms present in honey from phyla to species from each location. The fungal Amplicon Sequence Variants (ASVs) were aggregated from phyla to species exhibiting an abundance exceeding 0.005% were selected for inclusion in the honey composition plot. Three prominent phyla, namely Ascomycota, Basidiomycota, and Mortierellomycota, were identified across all honey samples together with their class, order, and family for each honey sample. However, their composition and abundance exhibit variations in each specific honey sample. Notably, the fungal phylum Ascomycota was the sole taxonomic group shared among all samples from Lombok, Bali, and Banggi, whereas Basidiomycota were dominant in Bali and Bangi honey only (Supplementary file S1).
Honey fungal abundance plot at the Phyla/Class (Supplementary file S1), Order, Family, Genus/Species level. For every honey sample, ASVs were aggregated to the genus level. For this plot, we only used species with a relative abundance of more than 0.005% across all samples. Any genus and species less than 1% that not mentioned here can be found in Supplementary files S2 and S3.
At phylum level, all replicates of Lombok honey samples (B1) were dominated by the phylum Ascomycota (99.61%), followed by Basidiomycota (0.34%), while Mortierellomycota accounted for 0.04%. At the genus level, the Lombok honey samples were dominated by the genera Zygosaccharomyces, Aspergillus, and Aureobasidium, with relative read abundances of 68.81%, 29.76%, and 0.46%, respectively. At the species level, the predominant species was Zygosaccharomyces mellis, representing 68.55% of the total read abundance. Subsequently, Aspergillus penicillioides was identified as the second most dominant species with a relative read abundance of 27.66%, while Aspergillus arenarioides represented the third dominant species with a relative read abundance of only 1.19% of the total reads (Fig. 2). Similarly, the mycobiota of Bali (B2) honey primarily comprises Ascomycota (71.46%), followed by Basidiomycota (28.18%), with Mortierellomycota accounting for a minor fraction (0.37%). Analysis at the genus level reveals the presence of nine dominant genera in Bali (B2) honey samples. Genus Aureobasidium dominates with a relative read abundance of 40.81%, other genera like Aspergillus, Cystobasidium, Exophilia, Cerrana, Rhodotorula, Trichoderma, Penicillium, Phlebiopsis, Peniophora, Magnibotryascoma, Lulwoana, Tinctoporellus, Epithele, and Xylodon represent 1–5% of the relative read abundance. Further examination at the species level indicates that Aureobasidium pullulans is the predominant species in Bali (B2) samples, constituting 40.1% of the relative read abundance, other species like Cystobasidium calyptogenae, Exophiala oligosperma, Cerrana unicolor, Peniophora crassitunicata, Rhodotorula mucilaginosa, Peniophora malaiensis, Tinctoporellus bubalinus, Magnibotryascoma kunmingense, contribute minimally, each representing 1–5% of the relative read abundance (Fig. 2) (Supplementary file S3).
In comparison, the phylum-level analysis of Bali honey samples (B3) reveals a different composition, with Basidiomycota accounting for the majority 61.87%, followed by Ascomycota (37.7%), and Mortierellomycota (0.53%). Dominant genera Bali honey (B3) is represented by Aureobasidium (25.13%), second highest genera Cerrana (8.77%), other genera Zygosaccharomyces, Aspergillus, Cystobasidium, Exophilia, Rhodotorula, Phlebia, Trichoderma, Penicillium, Trametes, Tinctoporellus, Schizophyllum, Letendraea, Montagnula, Physisporinus, Hortaea, Trichomerium, Talaromyces, and Fomitopsis represent 1–5% of the relative read abundance. At level species, Aureobasidium pullulans remains the dominant species in Bali (B3) honey, constituting 25.13% of the relative read abundance, followed by Cerrana unicolor (8.77%). Notable contributions from other species, including Zygosaccharomyces rouxii, Cystobasidium calyptogenae, Exophiala oligosperma, Rhodotorula mucilaginosa, Penicillium echinulonalgiovense, Trametes cubensis, Tinctoporellus bubalinus, Schizophyllum commune, Letendraea cordylinicola, Hortaea werneckii, Trichomerium lapideum, Trametes elegans, and Gliomastix murorum, represent 1–5% of the relative read abundance (Fig. 2).
Comparative analysis between Bali (B2) and Bali (B3) honey samples reveals both overlap** and distinct characteristics in their mycobiota. While both samples share Aureobasidium pullulans as the dominant species, with varying relative abundances of 40.1% in (B2) and 25% in (B3) respectively, while differences in phylum-level dominance are evident, with Ascomycota being predominant in B2 and Basidiomycota in B3. Additionally, variations in the relative abundances of several genera and species are observed between B2 and B3. For instance, Rhodotorula is significantly more abundant in B3 (8.7%) compared to B2 (1%). These findings suggest that while both samples share microbial components, specific factors may influence their mycobiota composition. Factors such as floral sources, collection time, and storage conditions may contribute to the observed variations in microbial communities. Thus, despite their geographical proximity, B2 and B3 honey samples exhibit distinct mycobiota compositions, reflecting potential influencing factors unique to each sample.
In Banggi honey (B4) sample, the phyla Ascomycota dominates at reads abundance 90.09%, followed by Basidiomycota at 9.79% and Mortierellomycota with only 0.11% of relative read abundance. At genus level the Banggi honey over dominant by Zygosaccharomyces (45.17%), followed by 2nd highest genera Aureobasidium (35.24%). Other genera like Aspergillus, Cystobasidium, and Exophilia represent 1–2% of the relative read abundance. The predominant species in Banggi (B4), honey samples are highly dominant by Zygosaccharomyces rouxii (42.71%), followed by 2nd highest dominant species are Aureobasidium pullulans (35.49%). Other genus like Cystobasidium calyptogenae, Exophiala oligosperma, represent only 1–2% of absolute read abundance (Fig. 2).
The examination of honey samples obtained from Lombok, Bali, and Banggi demonstrates clearly differentiated fungal compositions. Although Ascomycota is found all over, Basidiomycota is only dominant in Bali and Banggi. The predominant species found in Lombok honey is Zygosaccharomyces mellis, along with the presence of Aspergillus penicillioides. These microorganisms, Zygosaccharomyces and Aspergillus penicillioides, are distinctive features of Lombok honey. Aureobasidium pullulans is the predominant microorganism found in Bali honey, while Zygosaccharomyces rouxii is the dominant microorganism in Banggi honey. These findings emphasize the impact of geographical factors on the variety of fungi found in honey, emphasizing the significance of comprehending changes in microbiota for purposes of quality control and health evaluation.
Methods
Collection of honey samples
The four honey samples were obtained from each origin (Table 3) harvested in the months of early August 2023 to mid of November 2023. The honey samples were collected in biological quadruplicates from each location. The freshly collected honey was labeled as follows: Lombok (B1), Bali (B2 & B3), and Banggi Island (B4) (Fig. 3). All honey samples were stored in screw-capped dark containers at room temperature (approximately 25 °C) prior to analysis.
eDNA preparation from honey
The extraction of DNA was performed with little modification as described previously2,3. A 50 g sample of honey was divided into four 50 mL Falcon tubes, each containing 12.5 g of honey. Subsequently, 2 mL of ultrapure water was added to each tube. The solutions were then incubated at a temperature of 65 °C for 30 min while being stirred. Following centrifugation at a force of 12,000 × g for 20 min, the liquid portions located above the mixture were removed and discarded. The pellet samples were resuspended and mixed in a solution consisting of 1 mL of ultrapure water and 1 mL of phosphate-buffered saline (PBS). The resulting mixture was then transferred to a 2 mL centrifuge tube and subjected to centrifuged at 12,000 × g for another 20 min. The liquid portion was disposed of, while the solid residue was preserved at a temperature of − 20 °C. For further DNA extraction experiments, the Zymobiomics DNA Extraction Kit (Zymo Research, CA, USA) was utilized following the manufacturer’s instructions.
Library preparation and sequencing
The fungal ITS1 region was amplified using the primers BITS (5′ACCTGCGGARGGATCA3′) and B58S3 (5′GAGATCCRTTGYTRAAAGTT3′)21,22. An additional 5 bases of inline barcode were incorporated at the 5’ end of the primers to enable inline barcoding. Different samples were amplified using different combinations of the forward and reverse inline primers. PCR was performed using SolarBio 2X Taq PCR MasterMix (SolarBio Life Sciences, Bei**g, China) with the PCR profile of 95 °C for 3 min followed by 35 cycles of 95 °C for 15 s, 48 °C for 20 s and 72 °C for 10 s with final extension at 72 °C for 5 min. The barcoded amplicons were subsequently visualized on an electrophoresis gel. In each experiment, the PCR amplification is deemed successful only when samples exhibit a discernible band of the correct size on the gel, and the negative control displays neither the anticipated band nor primer dimer and subsequently purified using 0.8 X of solid-phase reversible immobilization (SPRI) bead. The purified amplicons were used as the template for 8 cycles of index PCR to incorporate the complete Illumina adapter and Illumina-compatible dual-index barcodes. The constructed libraries were subsequently size selected using 0.8 X vol of SPRI bead and pooled into a single tube. Quantification of the pooled libraries used Denovix DsDNA High Sensitivity Assay (DeNovix Inc, DE, USA). Sequencing of the pooled libraries was performed on a NovaSEQ6000 (Illumina, San Diego, USA) using the 2 × 150 bp paired-end sequencing configuration.
Data analysis
Demultiplexing and primer trimming of the raw paired-end reads used cutadapt v1.1823. The trimmed reads were subsequently merged using fastp v0.2124. The processed reads were imported into QIIME2 v.2022.825 for further analysis. Amplicon Sequence Variants (ASVs) were obtained using the dada2 v1.22 R package26. Subsequently, potential contaminants were identified in silico from the dataset using the R package DECONTAM27. Despite applying a conservative threshold value of p < 0.10 to identify potential contaminants, the dataset under consideration met this criterion, and consequently, no taxa were excluded from the dataset. Taxonomic assignment of the ASVs was carried out using q2-feature-classifier by Bokulich et al.28, which has been trained on the latest UNITE database (unite_ver9_dynamic)29. Only ASVs with taxonomic assignment at least to the phylum level were selected for subsequent analysis. The ASV table and taxonomic classification table were exported using QIIME2 tools into tab-separated values (TSV format) and manually formatted to generate Microbiome Analyst-compatible inputS1), indicating a highly diverse fungal community at the genus level. Comparing honey samples at the genus level, several genera such as Aureobasidium, Aspergillus, Cystobasidium, Exophiala, Cerrena, Rhodotorula, Eurotiales, Phlebia, Trichoderma, Penicillium, Phlebiopsis, Lulwoana, and Trametes show high resemblance. Additionally, unique genera such as Peniophora, Epithele, Vishniacozyma, Cladophialophora, Magnibotryascoma, Steccherinum, Coriolopsis, Epicoccum, Scopuloides, Nigrograna, Corynespora, and Neooccultibambusa are found only in Bali honey samples (B2 and B3), distinguishing them from other samples (see supplementary file S2). At the genus level, Zygosaccharomyces is highly abundant only in Lombok and Green honey samples, indicating a healthy yeast growth environment. Previous studies have also isolated Zygosaccharomyces from honey using both culture-dependent and non-dependent methods34.
Aureobasidium a dominant genus in Bali (B2 and B3) and Banggi honey (B4), likely due to its presence in the phyllosphere and carposphere of various fruits and vegetables, which honeybees easily transfer. This is consistent with previous findings9,35. Notably, Aureobasidium is identified for the first time in Banggi honey (B4) sample. A xerophilic fungus, is highly abundant in Lombok honey (B1) compared to other samples, as it thrives in environments with low water activity36 (see supplementary file S3). Zygosaccharomyces rouxii and Zygosaccharomyces mellis are dominant species within the Zygosaccharomyces genus, with Zygosaccharomyces mellis being highly abundant in Lombok honey (B1) and Zygosaccharomyces rouxii in Banggi honey (B4). The diversity observed in fungal communities across honey samples highlights their varied origins and hosts, corroborated by existing literature1,3,13.
The presence of Zygosaccharomyces, Aureobasidium, and Aspergillus in honey samples is not only common in the current study but also widely reported in other literature1,8,9. This prevalence can be attributed to their ability to tolerate the unique environmental conditions of honey. These organisms possess traits such as osmotolerance, xerotolerance, and acidotolerance, which allow them to thrive in environments characterized by high sugar content, low water activity, and low pH, respectively. Consequently, they are well-suited for survival and proliferation in honey, which typically exhibits these properties. Zygosaccharomyces, Aureobasidium, and Aspergillus are known to inhabit various natural environments, including soil, plants, and decaying organic matter, suggesting their adaptability to diverse ecological niches, including the floral sources of honey. It is believed that these organisms may enter honey through nectar sources. During the collection of nectar from flowers, bees may inadvertently transport fungal spores present in the environment, which subsequently contaminate honey during the production process. Aureobasidium and Aspergillus are sometimes associated with honey contamination. External sources, such as the bee foraging environment, can introduce fungal spores into honey from decaying plant matter, leading to contamination during harvesting and processing. The use of common metagenomic analysis worldwide has facilitated the application of culture-independent methods, such as metagenomic analysis, in studying the microbial composition of honey. This approach has revealed the presence of these fungi in honey samples, even at low abundance, thereby offering a more comprehensive understanding of the microbial community present in honey. Despite the valuable insights, certain limitations must be acknowledged. The sample size was limited due to the restricted number of reliable honey producers in Bali, Lombok, and Banggi Island, and the unique green honey (B4) from Sabah is found only on Banggi Island. These constraints limited the number of samples that could be collected and analyzed. Additionally, the collection timeframe, from early August 2023 to mid-November 2023, represents a specific period within the honey production season. Consequently, the findings provide a snapshot rather than a comprehensive analysis of the temporal dynamics of the honey microbiome.
Conclusion and future perspective
Current study demonstrates the effectiveness of ITS1 analysis in accurately distinguishing honey samples from different regions, highlighting its potential as a valuable tool for honey authentication. The findings affirm the superiority of DNA-based identification methods over traditional techniques. Notably, honey samples from Bali exhibit significantly higher levels of alpha and beta diversity compared to samples from other regions, highlighting a rich and unique microbial composition. These results emphasize the importance of geographical variation in honey quality and the need for precise authentication methods in the honey industry. The distinct microbial profile of Bali honey suggests unique environmental and floral influences. Recognizing the limitations of the current work future research should aim to include a larger and more diverse set of honey samples collected over extended periods and under varying environmental conditions. This approach will provide a more comprehensive understanding of the dynamic changes in the honey microbiome. Additionally, integrating physical and chemical analyses of honey samples will offer deeper insights into the factors influencing microbial diversity. These efforts will enhance in understanding of honey microbiomes and their implications for product quality and authenticity in the honey industry.
Data availability
Sequence data for this study were uploaded to the NCBI SRA database under Project (PRJNA1036045) with the following accession number: (a) SRX22424189 Lombok Honey (B1): Fungal species identified in honey samples from Lombok Island, Indonesia. (b) SRX22424192 Bali Honey (B2): Fungal species identified in honey samples from Bali Island, Indonesia. (c) SRX22424192 Bali Honey (B3): Fungal species identified in honey samples from Bali Island, Indonesia. (d) SRX22424191 Banggi Honey (B4): Fungal Species identified in Honey samples from Banggi Island, Sabah.
References
Balzan, S. et al. Microbial metabarcoding highlights different bacterial and fungal populations in honey samples from local beekeepers and market in north-eastern Italy. Int. J. Food Microbiol. 334, 108806 (2020).
Bovo, S. et al. Shotgun metagenomics of honey DNA: Evaluation of a methodological approach to describe a multi-kingdom honey bee derived environmental DNA signature. PLoS ONE 13, e0205575 (2018).
Wirta, H., Abrego, N., Miller, K., Roslin, T. & Vesterinen, E. DNA traces the origin of honey by identifying plants, bacteria and fungi. Sci. Rep. 11, 1–14 (2021).
Yan, S. et al. Natural plant toxins in honey: An ignored threat to human health. J. Hazard. Mater. 424, 127682 (2022).
Ra**dran, N. et al. Physicochemical properties of a new green honey from Banggi Island, Sabah. Molecules 27, 4164 (2022).
Lani, M. N., Zainudin, A. H., Razak, S. B. A., Mansor, A. & Hassan, Z. Microbiological quality and pH changes of honey produced by stingless bees, Heterotrigona itama and Geniotrigona thoracica stored at ambient temperature. Malays. Appl. Biol. 46, 89–96 (2017).
Donovan, P. D., Gonzalez, G., Higgins, D. G., Butler, G. & Ito, K. Identification of fungi in shotgun metagenomics datasets. PLoS ONE 13, e0192898 (2018).
**ong, Z. R., Sogin, J. H. & Worobo, R. W. Microbiome analysis of raw honey reveals important factors influencing the bacterial and fungal communities. Front. Microbiol. 13, 1099522 (2023).
Wen, Y. et al. The microbial community dynamics during the vitex honey ripening process in the honeycomb. Front. Microbiol. 8, 1649 (2017).
Jensen, A. B. et al. Standard methods for fungal brood disease research. J. Apic. Res. 52, 1–20. https://doi.org/10.3896/IBRA.1.52.1.13 (2013).
Schwarz, R. S., Huang, Q. & Evans, J. D. Hologenome theory and the honey bee pathosphere. Curr. Opin. Insect Sci. 10, 1–7. https://doi.org/10.1016/j.cois.2015.04.006 (2015).
Rutkowski, D., Weston, M. & Vannette, R. L. Bees just wanna have fungi: A review of bee associations with nonpathogenic fungi. FEMS Microbiol. Ecol. 99, fiad077 (2023).
Bovo, S., Utzeri, V. J., Ribani, A., Cabbri, R. & Fontanesi, L. Shotgun sequencing of honey DNA can describe honey bee derived environmental signatures and the honey bee hologenome complexity. Sci. Rep. 10, 1–17 (2020).
Engel, P., Martinson, V. G. & Moran, N. A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. 109, 11002–11007. https://doi.org/10.1073/pnas.1202970109 (2012).
Hamdi, C. et al. Gut microbiome dysbiosis and honeybee health: Gut microbiome dysbiosis and honeybee health. J. Appl. Entomol. 135, 524–533. https://doi.org/10.1111/j.1439-0418.2010.01609.x (2011).
Raymann, K., Coon, K. L., Shaffer, Z., Salisbury, S. & Moran, N. A. Pathogenicity of Serratia marcescens strains in honey bees. mBio 9, e01649-e1618. https://doi.org/10.1128/mBio.01649-18 (2018).
Anderson, K. E. et al. Microbial ecology of the hive and pollination landscape: Bacterial associates from floral nectar, the alimentary tract and stored food of honey bees (Apis mellifera). PLoS ONE 8, e83125. https://doi.org/10.1371/journal.pone.0083125 (2013).
Disayathanoowat, T. et al. Different dynamics of bacterial and fungal communities in hive-stored bee bread and their possible roles: A case study from two commercial honey bees in China. Microorganisms 8, 264. https://doi.org/10.3390/microorganisms8020264 (2020).
Powell, J. E., Martinson, V. G., Urban-Mead, K. & Moran, N. A. Routes of acquisition of the gut microbiota of the honey bee Apis mellifera. Appl. Environ. Microbiol. 80, 7378–7387. https://doi.org/10.1128/AEM.01861-14 (2014).
Yun, J. H., Jung, M. J., Kim, P. S. & Bae, J. W. Social status shapes the bacterial and fungal gut communities of the honey bee. Sci. Rep. 8, 2019. https://doi.org/10.1038/s41598-018-19860-7 (2018).
Bokulich, N. A. & Mills, D. A. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl. Environ. Microbiol. 79, 2519–2526 (2013).
Usyk, M., Zolnik, C. P., Patel, H., Levi, M. H. & Burk, R. D. Novel ITS1 fungal primers for characterization of the mycobiome. mSphere 2(6), e00488-17. https://doi.org/10.1128/mSphere.00488-17lenn (2017).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Davis, N. M., Proctor, D. M., Holmes, S. P., Relman, D. A. & Callahan, B. J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6, 1–14 (2018).
Bokulich, N. A. et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6, 1–17 (2018).
Tedersoo, L. et al. The global soil mycobiome consortium dataset for boosting fungal diversity research. Fungal Divers. 111, 573–588 (2021).
Chong, J., Liu, P., Zhou, G. & **a, J. Using Microbiomeanalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 15, 799–821 (2020).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, 1–18 (2011).
Ondov, B. D., Bergman, N. H. & Phillippy, A. M. Interactive metagenomic visualization in a web browser. BMC Bioinform. 12, 1–10 (2011).
Walters, K. E. & Martiny, J. B. Alpha-, beta-, and gamma-diversity of bacteria varies across habitats. PLoS One 15, e0233872 (2020).
Ullah, S. et al. Baseline amplicon sequencing data for the ITS2 region in the green honey of Banggi Island, Sabah. Data Brief 52, 110044. https://doi.org/10.1016/j.dib.2024.110044 (2024).
Iqbal, M. et al. Bee-vectored Aureobasidium pullulans for biological control of gray mold in strawberry. Phytopathology® 112, 232–237 (2022).
Nazareth, S. & Gonsalves, V. Aspergillus penicillioides—a true halophile existing in hypersaline and polyhaline econiches. Anna. Microbial. 64, 397–402 (2014).
Acknowledgements
The authors extend sincere appreciation to the private suppliers: Madu Lombok Utara in Desa Sukadana, North of Lombok; Raw Bali honey from Honeybee Farms in Desa Kuwum, Badung, and Tenganan, Manggis, Karangasem Regency, Bali; and NS Field Sdn Bhd for providing green honey from Banggi Island, Sabah. Gratitude is also extended to Udayana University for their generous support through the UNISERF Program (BRIN), which provided research funding (Reference number: B/775-4/UN14.4A/PT.01.03/2023). Their invaluable assistance and support significantly contributed to the successful completion of this research.
Author information
Authors and Affiliations
Contributions
S. U.: data curation, formal analysis, methodology, investigation, software, writing—original draft; F. H.: supervision, funding acquisition, project administration, validation, writing—review & editing; R. A. W.: supervision, validation, resources, writing—review & editing; N. H.: conceptualization, data curation; writing—review & editing; H. A. O.: resources, writing—review & editing; I. G. A. S.: resources, writing—review & editing; S. S.: conceptualization, methodology, data curation; A. A. S. P. R. A.: resources, writing—review & editing; M. A. N. M.: conceptualization, methodology, data curation; writing—review & editing; A. A. A. H.: conceptualization, methodology, data curation; M. H. M. N.: resources, writing—review & editing; N. S. A.: resources, writing—review & editing; I. B. W. G.: supervision, funding acquisition, project administration, conceptualization, methodology, data curation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ullah, S., Huyop, F., Wahab, R.A. et al. The first ITS1 profiling of honey samples from the Southeast Asian region Lombok, Bali and Banggi Island. Sci Rep 14, 14122 (2024). https://doi.org/10.1038/s41598-024-64838-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64838-3
- Springer Nature Limited