Abstract
Patients with cervicogenic headache (CeH) showed lower spinal postural variability (SPV). In a next step, the complex character of such SPV needs to be analysed. Therefore, variables influencing SPV need to be explored. A non-randomized repeated-measure design was applied to analyse relations between biopsychosocial variables and SPV within a CeH-group (n = 18), 29–51 years, and matched control-group (n = 18), 26–52 years. Spinal postural variability, expressed by standard deviations, was deducted from 3D-Vicon motion analysis of habitual spinal postures (degrees). Interactions between SPV and pain processing, lifestyle, psychosocial characteristics were analysed. Pain processing characteristics included symptoms of central sensitization (Central Sensitization Inventory), (extra)-cephalic pressure pain thresholds (kPa/cm2/s). Lifestyle characteristics included sleep quality (Pittsburgh Sleep Quality Index), physical activity, screen-time, sedentary-time (hours a week), position (cm) and inclination (degrees) of the laptop (= desk-setup). Psychosocial characteristics included degree of depression, anxiety and stress (Depression Anxiety Stress Scale-21), impact of headache on quality of life (Headache Impact Test-6). Spinal postural variability related significantly to intrinsic (stress, anxiety, extra-cephalic pressure pain thresholds, sleep-duration) and extrinsic (desk-setup, screen-time) variables in the CeH-group. In the control-group, SPV related significantly to extra-cephalic pressure pain thresholds. Spinal postural variability related to diverse variables in the CeH-group compared to the control-group. More research is needed into a possible causal relationship and its clinical implication.
Similar content being viewed by others
Introduction
Motor variability is hypothesized to fulfil a beneficial role in preventing the development of overuse injuries and pain1. Its dynamic spectrum ranges from subtle motor adaptations, to complete avoidance of movement as response to (impending) pain2,3,4. However, the relationship between pain and motor variability is complex. Too little or too much variability could harm tissue2. Secondly, motor responses to pain can be highly variable between, and probably within individuals due to the unique character of motor variability3,5,6.
Although motor variability can be individual- and task-specific driven, generally accepted theories predict rather stereotypical motor responses to pain7,8. The pain adaptation theory supports decreased activity of muscles producing painful movements, and facilitation of antagonists7. The pain-spasm-pain cycle or vicious cycle theory on the other hand, predicts increased muscular activity with at the end more pain induced by the process of muscle ischaemia8. A more contemporary theory of motor responses to pain postulates that pain may force a patient to adopt a protective and less variable posture3,4. This contemporary theory suggests that pain aims to protect the painful or threatened part of the body by constraining movement. Falla et al. demonstrated that such protective motor adaptations even occur at a distance from the site of pain, with participants with neck pain walking with a stiffer (i.e. decreased rotation) trunk3,9. It is hypothesized that the overall goal of this protective behaviour is to enhance stability by resisting intervertebral perturbations3,10. Understanding motor variability in the context of pain could certainly guide management for recovery and restoration of function2. In this regard, motor variability is already studied in patients suffering from work-related musculoskeletal overuse injuries, low back pain, and recently cervicogenic headache (CeH)1,4,5,6,8,11. Recently lower motor variability during laptop-work, referred to as spinal postural variability (SPV), was demonstrated in patients with CeH11.
Focusing on postural research in CeH might become of special interest within the current context of COVID-1912. Prevalence of CeH might increase as a consequence of COVID-19. Two separate pathways could support such hypothesis: (1) indirectly, due to the forced ‘working from home’ policy resulting in a less optimal ergonomic work-setting, and (2) directly, due to being more sedentary in being sick and/or recovering from the COVID-19 infection. Both pathways might relate to less optimal and less variable postural behaviour, potentially contributing to develo** CeH13,14,15.
As a consequence, it might be hypothesized that decreased cervical SPV, due to stereotypical motor behaviour and maintaining a posture1,16, might contribute to CeH.
Though research has been performed to analyse SPV in patients with CeH, research into biopsychosocial (BPS) variables such as pain, psychosocial, and lifestyle characteristics, influencing such variability is not available for this population11. Identifying such variables could attribute to a better understanding of the complex multi-dimensional character of SPV, its relation to CeH and how to prevent/manage it in the future.
Therefore, the main objective of the current study was to explore if BPS variables such as pain, psychosocial, and lifestyle characteristics significantly relate to SPV in patients with CeH compared with healthy controls.
Methodology
Design
Non-randomized analysis of relations between BPS variables and SPV during the 30-min-laptop-task within the CeH-group and matched control-group.
Sample size
An a priori sample size was estimated (G*Power 3.1.9.4, Kiel, Germany) based on repeated-measures at five time-points (F-test, between-factors) of the forward head posture (FHP) (mean degrees and standard deviation) during a laptop-task. A total of 30 participants (15 participants per group, power 80%; α = 0.05) was required to detect a mean difference of 3.5° (± 1.3) in FHP between the headache-group and control-group17.
Participants and ethics
Participants for both the CeH-group and the control-group were recruited between January 2018 and August 2019. The neurological staff at the headache departments of the AZ Vesalius hospitals (Tongeren and Bilzen, Belgium) identified and referred participants meeting the study’s inclusion criteria for CeH (see below for details). Additionally, a general call was launched at the Hasselt University, Zuyd Hogeschool (The Netherlands) and physiotherapy practices. Each potential participant for the CeH-group had to be declared eligible by a neurologist (external member of the research team). The neurologist was involved in determining the inclusion and exclusion criteria, confirmed the diagnosis of CeH based on the International Classification of Headache Disorders-3 (ICHD-3) criteria18, and referred eligible participants with CeH to the principal researcher (= physiotherapist, degree in manual therapy, > 10 years of experience).
Potential participants for the control-group were recruited by convenience sampling, word-of-mouth advertising within the Zuyd Hogeschool, and in the personal network of the involved researcher (Appendix A, Figure A.1).
Inclusion criteria for the CeH-group were: Dutch-speaking participants between 18 and 55 years, body mass index (BMI) between 18.5 and 24.9 kg/m2, diagnosed with secondary episodic CeH according to the ICHD-318 by a neurologist, normal cognitive capacity (Mini Mental State Examination test score of 30), habitual laptop-use (minimum of 7 h/week) (Appendix A, Table A.1). Inclusion criteria for the control-group were: Dutch-speaking healthy participants (i.e. no known painful conditions or serious pathologies) between 18 and 55 years, BMI between 18.5 and 24.9 kg/m2, normal cognitive capacity (Mini Mental State Examination test score of 30), habitual laptop-use (minimum of 7 h/week) (Appendix A, Table A.1). Exclusion criteria for both groups were: pregnancy, physiotherapy for head- or neck-related disorders in the past month before the start of the study, serious pathology (musculoskeletal, neurological, endocrine, cardiovascular, psychiatric), medication overuse (intake of NSAID’s, opioids, acetylsalicylic acid, triptans, simple analgesics for > 10 days/month > 3 months), smoking, history of neck/head trauma, orthodontics (Appendix A, Table A.1).
Nineteen participants were recruited and selected to compose the CeH-group (Appendix A, Figure A.1). These participants were given a four-week headache-diary which had to be completed before the start of the study. The control-group was matched for age, gender, ethnicity and socio-economic status (level of education, job).
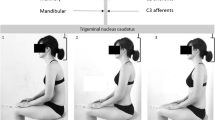
The current study was part of phase 1 of a larger project (hence the larger sample size) which was registered as an observational study at ClinicalTrials.gov (registered on 02/09/2016, NCT02887638). The Medisch Ethische Toetsings Commissie of Zuyderland and Zuyd Hogeschool (NL. 55720.09615) and the Comité Medische Ethiek of the Ziekenhuis Oost-Limburg (B371201423025) granted approval to execute the experimental protocol. Eligible participants had to read and sign the informed consent before officially being enrolled. Protection of personal data was legally determined by the Belgian law of December 8th 1992. All test procedures involving human participants were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki Declaration and its later amendments. An informed consent was obtained from the participant in Fig. 4 for publication of the identifying image. The current study was prepared according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Measurements, outcomes and instruments
Primary outcome: spinal postural variability (SPV)
Spinal postural variability expresses variation in habitual spinal posture (= the position of the spine expressed in degrees) of the upper- and lower-cervical (UCx, LCx), thoracic (UTx, LTx), and lumbar (ULx, LLx) spine. Habitual spinal postures were evaluated with a 3D-Vicon motion analysis system (Vicon Motion Systems Ltd., Oxford, UK) and Vicon Nexus software (version 2.1.1, Oxford Metrics Ltd., Oxford, UK)19 for recording, data acquisition, storage, and gap filling during the 30-min-laptop-task11,20,21. The accuracy of the system is < 1° and < 1.5° root mean square in static and dynamic angular measurements, respectively20,21,22. Spinal postural variability of the UCx, LCx, UTx, LTx, ULx, and LLx, expressed by the standard deviation (SD), was deducted from the habitual spinal postures during the 30-min-laptop-task.
Secondary outcomes: biopsychosocial (BPS) variables
Interpretations of the outcomes are presented in Appendix B (Tables B.1 to B.5).
Pain processing characteristics
-
1.
Pressure Pain Thresholds (PPTs) (kPa/cm2/s) of the bilateral suboccipitals (cephalic), erector spine at L1 (extra-cephalic), and tibialis anterior (extra-cephalic) were measured with an electronic pressure algometer (Somedic AB, Stockholm, Sweden)23,24,25,26,27. Pressure Pain Threshold is defined as the minimal amount of pressure that elicits pain. Hypersensitivity over remote, extra-cephalic sites is considered to be a sign of central sensitization. Intra-rater reliability of cervical PPT-measurements are moderate to good (ICC 0.79–0.90) in healthy participants, and good to excellent (ICC 0.82–0.99) in patients with headache24,26,28. Intra-rater reliability of erector spine PPT-measurements are excellent (ICC 0.95–0.98) in healthy participants29. Intra-rater reliability of tibialis anterior PPT-measurements are excellent in healthy participants (ICC 0.94), and patients with neck pain (ICC 0.97)24.
-
2.
Symptoms of central sensitization were questioned via the Dutch Central Sensitization Inventory (CSI)30. Test–retest reliability (ICC 0.82–0.97), and internal constancy (Cronbach’s α 0.87–0.91) are good to excellent31,32.
-
3.
Headache characteristics, i.e. headache-intensity (mean 100 mm Visual Analogue Scale (VAS) per attack during a month), duration (mean hours per attack during a month), and frequency (days per month) were extracted from the headache diary (Belgian Headache Society)33. Additional information on referred pain from the neck (yes/no) was obtained through the anamnesis.
The Numeric Pain Rating Scale (NPRS) was used to capture headache-intensity pre and post the 30-min-laptop-task34,35,36,37.
Lifestyle characteristics
-
1.
Sleep quality was assessed via the Dutch Pittsburgh Sleep Quality Index (PSQI) which is a standardized, valid and reliable self-reported 1-month recall questionnaire38,39,40,41. The index differentiates poor from good sleepers by measuring seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of slee** medication, and daytime dysfunction.
-
2.
Physical activity (mean hours a week), screen-time (mean hours a week of computer-use), and sedentary-time at home and during work (mean hours a week) were extracted from a customized one-week recall questionnaire.
Psychosocial characteristics
-
1.
Degree of depression, anxiety and/or stress was estimated by the Dutch Depression Anxiety Stress Scale-21 (DASS-21), a self-reported one-week recall questionnaire. Internal constancy for the three subscales is good to excellent (Cronbach’s α 0.91, 0.84, and 0.90, respectively)42,43,44. Each of the three sub-scales contain seven items. The depression subscale assesses dysphoria, hopelessness, devaluation of life, self-deprecation, lack of interest, anhedonia and inertia. The anxiety subscale estimates autonomic arousal, skeletal muscle effects, situational anxiety, and subjective experience of anxious affect. The stress subscale evaluates difficulty in relaxing, nervous arousal, and being easily upset and impatience.
-
2.
Impact of headache on quality of life was assessed with the Dutch Headache Impact Test-6 (HIT-6)45,46,47,48. Internal consistency is good to excellent (Cronbach’s α 0.89–0.90)46. The HIT-6 evaluates the impact of headache on daily activities: ability to function at work, school, home, and in social situations.
Procedure
A condition to be measured was a score of < 3 on the 11-point NPRS for headache-intensity on the test day3. Participants were asked not to take analgesics, muscle relaxants, and caffeine-containing beverages 24 h prior to the measurements. Prophylactic treatment(s) remained unchanged. Measurements were performed in a real-life set-up with a constant room temperature of 25°C at the motion laboratory of Zuyd Hogeschool (Heerlen, The Netherlands), and executed by the principal researcher.
Questionnaires were completed first, followed by PPT-measurements, and final habitual spinal posture measurements (Fig. 1). Headache-intensity and neck pain intensity (NPRS) were questioned pre and post the 30-min-laptop-task in both groups.
Chronology of the test procedure for both groups. We refer to our previous work and Appendix C concerning the SPV-measurements (grey box)11 (NPRS = Numeric Pain Rating Scale, CSI = Central Sensitization Inventory; PSQI = Pittsburgh Sleep Quality Index; DASS = Depression, Anxiety, Stress Scale; HIT = Headache Impact Test; PPT = Pressure Pain Threshold; SPV = Spinal Postural Variability) (Microsoft PowerPoint 2016, version 1, Microsoft Corporation).
A standardized protocol was used to measure PPTs of the bilateral suboccipitals, erector spine at L1 (neutral prone position) and tibialis anterior (seated with 80° knee-flexion)24,25. Pressure was perpendicular applied directly on the muscle belly, staring at 0 to maximal 1000 kPa, using a 1 cm2 probe with a slope of 30 kPa/s49,50. Participants were instructed to push the stop-button when the sensation of pressure changed into pain. An exercise trial was once performed on the right thigh before actually measuring. Measurements were executed twice (ICC 0.86–0.99) after a 5-min interval in a standardised column-wise order: suboccipital left, erector spine at L1 left, tibialis anterior left, suboccipital right, erector spine at L1 right, tibialis anterior right26,50,51,52. Mean values of two measurements on each location were used for further statistical analysis24,53.
Spinal postural variability of the UCx, LCx, UTx, LTx, ULx, and LLx during the 30-min-laptop-task were measured according to procedures described in our previous work11 (Appendix C). Figure 2 provides a visualization of the marker-placement used to determine UCx, LCx, UTx, LTx, ULx, and LLx angles. Spinal postural variability was deducted from the angular measurements.
Statistics
Analysis was done via JMP Pro 14 and SAS 9.4. Two-tailed tests at 5% level of significance were reported.
Demographics and group characteristics
Unpaired t-tests were used to compare continuous variables. Contingency tables (Fisher’s exact test) were composed to compare distributions of categorical variables (proportions) between groups.
Spinal postural variability was deducted from linear mixed models for repeated measures, based on the lowest root-mean-square error, with dependent variables (habitual spinal posture), fixed (time, baseline, group), random (individual, time) effects, and an autoregressive covariance structure [AR(1)]: Yij = β0 + β1X1ij + ⋯ + βkXkij + μi + εij (Appendix D, Table D.1).
Spinal postural variability was deducted from the random-intercept of this mixed model. To express SPV, mean SDs were calculated per habitual spinal posture (UCx, LCx, UTx, LTx, ULx, LLx) per group at t0 (since, based on the mixed model, time was not related to SPV) (Appendix D, Table D.2). Effect sizes (ES) to quantify differences in SPV between groups (Cohen’s d) were reported and interpreted as: ≤ 0.20 small, 0.21–0.49 moderate, 0.50–0.79 medium, ≥ 0.80 large ES54.
Next, multiple regression models with a stepwise approach were built per group to identify possible BPS dependent variables related to the SDs of the UCx, LCx, UTx, LTx, ULx, and LLx. Dependent variables were ranked from most to least influence based on R2 and standardized coefficients in case of model significance. Conditions to apply linear models (i.e. normal distribution, linearity, homoscedasticity of residuals, no multicollinearity (Variance Inflation Factor (VIF) < 4.00) between variables) had to be met.
Relations between the independent variables age, BMI, the interaction between age and BMI (age*BMI), headache-intensity (continuous), socioeconomic status (categorical), and SDs of the UCx, LCx, UTx, LTx, ULx, LLx (dependent continuous) were evaluated via multiple linear regression: Yi = β0 + β1Xi1 + β2Xi2 + β3Xi1Xi2 + ⋯ + βkXik + εi (Appendix E, Tables E.1 and E.2). Conditions to apply linear models had to be met.
Mean (SD) headache-intensity (NPRS) was compared pre and post the laptop-task by using the paired t-test. Effect sizes to quantify differences in change of headache-intensity from pre- to post-laptop-task were reported (interpretation see above)54.
Results
Demographics and group characteristics (Table 1)
Demographics and group characteristics were comparable between the groups. Two participants (one in the CeH-group, one in the control-group) had to be excluded because of technical artefacts during the postural measurements. Age, BMI, the interaction between age and BMI (age*BMI), level of education, employment and headache-intensity did not significantly relate to SPV (Appendix E, Tables E.1 and E.2).
Headache characteristics (Table 2)
Participants with CeH suffered from an episodic, moderate to severe intense headache with a mean duration of 4.1 h/episode. Referred pain from the neck was reported by all 18 participants. No headache or neck pain were reported at the start of the test procedure.
Mean headache-intensity (SD) was significantly (p < 0.001, ES 0.89) higher at the post laptop-task measurement [NPRS 3.6 (2.1)] compared to at the measurement before the laptop-task [NPRS 0.7 (1)]. This difference is larger than the minimal clinical meaningful difference of 2.537. Participants in the control-group did not report headache or neck pain pre and post-laptop task.
Spinal postural variability
Evolution of SPV during the 30-min-laptop-task (Fig. 3)
Figure 3 visualises fluctuations of SDs of the UCx, LCx, UTx, LTx, ULx, and LLx per minute during the 30-min-laptop-task in the CeH-group (grey) and control-group (black). The evolution of SDs was rather linear (time-effect p > 0.05, Appendix D, Table D.2), meaning that time did not relate to SPV. Mean SDs were generally lower in the CeH-group compared to the control-group11.
Comparison of SPV between the CeH-group and control-group (Table 3)
Spinal postural variability of the LLx was significantly lower (p < 0.001) in the CeH-group compared to the control-group, which indicated that these participants moved less during the 30-min-laptop-task. Spinal postural variability of the other spinal postures was, however not significant, consistently lower in the CeH-group compared to the control-group. Spinal postural variability of the ULx was significantly lower compared to LLx in both the CeH-group and control-group (p = 0.034, p < 0.001, respectively), indicating that participants moved less at the ULx compared to the LLx.
Relation between BPS variables and SPV
Detailed results on pain processing, psychosocial and lifestyle characteristics are presented in Appendix F (Tables F.1 and F.2).
Biopsychosocial variables that were significantly related to SPV in the CeH-group are summarized below (x represents the dependent variable in the equation). Interpretation of these results is visualized in Fig. 4:
-
Spinal postural variability of UCx decreased significantly if the level of stress increased (14.5–9.71x, p = 0.002), and sleep quality decreased (12.64–7.99x, p = 0.041).
-
Spinal postural variability of LCx decreased significantly with an increase in stress level (6.18–1.97x, p = 0.038), and in PPTs of the tibialis anterior left (14.46–0.02x, p < 0.001), SPV of LCx increased significantly with increasing sleep duration (− 7.69 + 1.99x, p = 0.030), and if the laptop position was further referred to the table-edge (x1) with a larger inclination of the laptop-screen (x2) (− 30.59 + 0.42x1 + 0.28x2, VIF 1.19, p = 0.036).
-
Spinal postural variability of UTx decreased significantly with increasing PPTs of the tibialis anterior left (6.79 – 0.008x, p = 0.025).
-
Spinal postural variability of LTx decreased significantly with increasing PPTs of the tibialis anterior right (6.25 – 0.007x, p = 0.044), and levels of stress (5.69 – 2.59x, p = 0.020), SPV of LTx increased significantly with an increase in level of anxiety (3.35 + 2.34x, p = 0.010).
-
Spinal postural variability of ULx increased significantly if the laptop was further positioned referred to the table edge (1.48 + 0.15x, p = 0.016).
-
Spinal postural variability of LLx decreased significantly with increasing screen-time (6.68 – 2.8x, p = 0.007).
Summary of significant relations between BPS variables and SPV in the CeH-group (tib ant = tibialis anterior; L = Left; R = Right; PSQI = Pittsburgh Sleep Quality index; Arrows ↓ and ↑ refer to the direction of the relation, namely decrease (less movement) and increase (more movement), respectively).
Biopsychosocial variables that were significantly related to SPV in the control-group are summarized below (x represents the dependent variable in the equation):
-
Spinal postural variability of LCx increased significantly with an increase in PPTs of the tibialis anterior right (− 10.56 + 0.05x, p < 0.001), and PPTs of the suboccipitals right (0.4 + 0.03x, p 0.002). Further, SPV of LLx increased significantly with increasing PPTs of the tibialis anterior right (− 3.29 + 0.03x, p = 0.002).
Discussion
The main objective of the current study was to explore SPV in patients with CeH from a BPS point of view. Although SPV of these patients is lower compared to healthy controls11, research into multi-dimensional variables related to such SPV is non-existent. Since motor variability results from continuous motor adaptations as response to individual demands of a task, pain or other multi-dimensional factors, a BPS approach seems essential to unravel the potential relationships with SPV in patients with this musculoskeletal pain condition55,56.
Lower spinal postural variability in patients with CeH
Motor variability (including among others SPV) was neglected for a long time by the scientific community. It was even referred to as ‘noise’ or ‘interference’57. More recently the role of motor variability retrieved attention in natural processes such as movement adaptation58,59. The latter should enable an individual to respond adequately to functional tasks and environmental constraints60, and prevent musculoskeletal overuse injuries and pain1,57,61. In general, it is presumed that higher motor variability is a healthier state, and lower motor variability relates to musculoskeletal disorders (MSDs). It should however be kept in mind that ‘too much’ motor variability can result in overuse-injuries62.
A renewed redundancy theory concerning motor variability was introduced based on the principle of abundance63. This theory postulates that there is no single optimal solution to resolve a task, but rather many similar solutions. This is referred to as ‘goal equivalent variability’59. Motor variability at both articular and muscular level, is an inherent characteristic of movement and is hypothesized to serve a protective role against develo** MSDs1,57,61,64,65. Within this view, it might be relevant to draw attention to the MSDs in CeH. Prevalence of MSDs of the upper-cervical spine, which can be triggers for CeH, increases in office workers who are intensive computer users66. And, as a result of the global pandemic caused by COVID-19, prevalence of CeH is expected to increase due to immobilisation and digitalisation (e.g. home office work)13. Cervicogenic headache is presumed to be related to rather static, forced and/or prolonged habitual (cervical) postures, which can increase load on cervical musculoskeletal structures innervated by C1-C3 afferents67,68,69,70. Although cervical posture (e.g. FHP) has been extensively studied in CeH, little research was performed regarding SPV11. Based on the redundancy theory, the general lower SPV in the CeH-group compared to the control-group may imply a less balanced state. Finding an equilibrium in SPV might reflect an individual’s self-regulatory capacity to function in an optimal range. Such equilibrium corresponds to a state of homeostasis, which is critical to the sustainability of living organisms71. Since the key factor in sustainability is variability, variables related to such variability need to be identified72.
Multiple biopsychosocial variables relate to spinal postural variability in patients with CeH
Previous studies already identified variables that influence intrinsic motor variability such as gender72,73, age74, experience (i.e. novice vs. experienced)67, fatigue75, musculoskeletal discomfort, and pain64,76. Several authors support the hypothesis that decreased motor variability is the motor response of patients suffering from long-term pain conditions (e.g. low back pain, unilateral patella-femoral pain, spastic hemiplegic cerebral palsy) in an attempt to limit movement as a protective mechanism77,78,79,80. However, we cannot confirm that headache-intensity was related to the lower SPV in the CeH-group. A first hypothesis for this finding could be the episodic character of CeH in our study, compared to chronic pain conditions in previous work77,78,79,80. Secondly, since cause-effect relations can be bidirectional, the lower SPV might have induced CeH.
The subjective individualized nature of experiencing pain, such as CeH, is created by a mix of factors unique to that person81. Both heterogeneity in clinical characteristics, and individual BPS characteristics such as gender, age, socio-economic status and psychosocial influences have been documented to influence the experience of pain, giving it a highly variable nature77,78,82. We extrapolated this knowledge to SPV, since we recently demonstrated that SPV also exists in patients with CeH11. The results of this current study revealed that SPV could be influenced by BPS variables in patients with CeH. Both intrinsic (stress, anxiety, sleep quality and pain processing) and extrinsic (desk-set-up, screen-time) variables seem to be significantly related to SPV.
Interestingly, several intrinsic and extrinsic BPS variables influenced SPV in the CeH-group, while LLx variability was the only outcome variable significantly different between groups. Two hypotheses might explain such difference in LLx variability. First, the lower LLx variability in the CeH-group (versus the control-group) might be the result of a protective strategy, and is consistent with the contemporary theory of motor adaptations to pain3. This theory suggests that pain induces postural adjustments to limit the amplitude of motion. Such adjustments can even occur at regions remote to the site of pain3. The results in our study could imply that participants with CeH are protecting their painful cervical spine by stiffening the lumbar region. Falla et al. (2017) already reported that participants with neck pain walked with a stiffer trunk9. Interestingly, in both studies a stiffening of the lumbar spine was seen9. This finding might indicate a bottom-up stiffening. However, future research is needed to analyse such hypothesis.
However, a second hypothesis should be considered. A minimum degree of variability is needed to create variation in joint load, muscle activity, and ligament stress as was postulated by the ‘variability-overuse hypothesis’3,4,57,83. The lower LLx variability in the CeH-group could place a cumulative load on the upper-cervical spine potentially activating a final common pathway (i.e. trigeminocervical nucleus caudatus) to induce CeH. More research is needed to further explore such finding, and its clinical relevance.
Time (the 30-min laptop-task) did not relate to SPV, implying that the course of SPV was rather flat and linear models (mean, SD) could be used84. Using non-linear models such as entropy analysis seems however needed to better understand the complexity of SPV85,86.
Clinical relevance
Although this study could identify relations between BPS variables and SPV in patients with CeH, its clinical implication needs further exploration. To date, it is questioned whether SPV is causally related to CeH and if an optimal range of SPV exists11,86,87. Optimal variability seems, similar to homeostasis, a state in which a posture tends to remain balanced and stable. Therefore, determining the limits of SPV could provide clinical essential cut-offs for the transition of a healthy state, towards discomfort and eventually pain76.
Limitations and recommendations
No Bonferroni corrections were applied because of the explorative nature. Therefore, and because of spectrum bias, results should be interpreted with caution.
Spinal postural variability can be assessed using several models. We used linear models (mean, SD) based on the characteristics of the data. It seems advised to additionally use non-linear models (e.g. entropy) to capture the complexity of SPV in future studies85,86. General residuals of the calibration method should have been provided to enable replications.
No cause-effect relationships between SPV and CeH can be extrapolated from the present analyses calling for longitudinal studies with adapted methodology, and possibly non-linear models.
Following these suggestions it should be questioned if a range of optimal variability exists, and if such range is specific to an individual80.
Conclusion
Significant relations between SPV and BPS variables add new insights to the current knowledge on CeH. Spinal postural variability was lower, and related to more diverse BPS variables (i.e. pain processing, lifestyle, and psychosocial) in the CeH-group compared to the control-group. More research is needed into a possible causal relationship between SPV and CeH, and its clinical implication for management of this condition.
Abbreviations
- BMI:
-
Body Mass Index
- BPS:
-
BioPsychoSocial
- CeH:
-
Cervicogenic headache
- CI:
-
Confidence interval
- CSI:
-
Central sensitization inventory
- DASS-21:
-
Depression anxiety stress scale
- ES:
-
Effect size
- FHP:
-
Forward head posture
- HIT-6:
-
Headache impact test
- ICC:
-
Intraclass correlation coefficient
- ICHD:
-
International classification of headache disorders
- IQR:
-
Inter quartile range
- L:
-
Left
- LCx:
-
Lower-cervical spine
- LLx:
-
Lower-lumbar spine
- LTx:
-
Lower-thoracic spine
- n:
-
Number of participants
- min:
-
Minute
- MSD(s):
-
Musculoskeletal disorder(s)
- N/A:
-
Not applicable
- NPRS:
-
Numeric pain rating scale
- PPT(s):
-
Pressure pain threshold(s)
- PSQI:
-
Pittsburgh sleep quality index
- R:
-
Right
- SD:
-
Standard deviation
- SPV:
-
Spinal postural variability
- UCx:
-
Upper-cervical spine
- ULx:
-
Upper-lumbar spine
- UTx:
-
Upper-thoracic spine
- VAS:
-
Visual analogue scale
- VIF:
-
Variance inflation factor
References
Srinivasan, D. & Mathiassen, S. E. Motor variability in occupational health and performance. Clin. Biomech. 27, 979–993 (2012).
Hodges, P. W. & Smeets, R. J. Interaction between pain, movement, and physical activity: Short-term benefits, long-term consequences, and targets for treatment. Clin. J. Pain 31, 97–107 (2015).
Hodges, P. W. & Tucker, K. Moving differently in pain: A new theory to explain the adaptation to pain. Pain 152, S90–S98 (2011).
Hodges, P. et al. Changes in the mechanical properties of the trunk in low back pain may be associated with recurrence. J. Biomech. 42, 61–66 (2009).
Hodges, P. W. et al. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp. Brain Res. 151, 262–271 (2003).
van Dieen, J. H., Selen, L. P. & Cholewicki, J. Trunk muscle activation in low-back pain patients: An analysis of the literature. J. Electromyogr. Kinesiol. 13, 333–351 (2003).
Lund, J. P. et al. The pain adaptation model: A discussion of the relationship between chronic musculoskeletal pain and motor activity. Can. J. Physiol. Pharmacol. 69, 683–694 (1991).
Roland, M. A critical review of the evidence for a pain-spasm-pain cycle in spinal disorders. Clin. Biomech. 1, 102–109 (1986).
Falla, D. et al. People with chronic neck pain walk with a stiffer spine. J. Orthop. Sports Phys. Ther. 47, 268–277 (2017).
Reeves, N. P., Narendra, K. S. & Cholewicki, J. Spine stability: The six blind men and the elephant. Clin. Biomech. 22, 266–274 (2007).
Mingels, S., Dankaerts, W., van Etten, L., Bruckers, L. & Granitzer, M. Lower spinal postural variability during laptop-work in subjects with cervicogenic headache compared to healthy controls. Sci. Rep. 11, 5159 (2010).
Borges do Nascimento, I.J. et al. Novel coronavirus infection (COVID‐19) in humans: A sco** review and meta‐analysis. J Clin Med. 9, E941 (2020).
Belvis, R. Headaches during COVID-19: My clinical case and review of the literature. Headache 60, 1422–1426 (2020).
Gerr, F., Monteilh, C. P. & Marcus, M. Keyboard use and musculoskeletal outcomes among computer users. J. Occup. Rehabil. 16, 265–277 (2006).
Gerr, F. et al. A prospective study of computer users: I. study design and incidence of musculoskeletal symptoms and disorders. Am J Ind Med. 41, 221–235 (2002).
Nordander, C. et al. Risk of musculoskeletal disorders among females and males in repetitive/constrained work. Ergonomics 52, 1226–1239 (2009).
Mingels, S., Dankaerts, W., van Etten, L., Bruckers, L. & Granitzer, M. Comparative analysis of head-tilt and forward head position during laptop use between females with postural induced headache and healthy controls. J. Bodyw. Mov. Ther. 20, 533–541 (2016).
Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 38, 1–211 (2018).
Vicon. https://www.vicon.com/software/ (2021).
Ehara, Y. et al. Comparison of the performance of 3D camera systems. Gait Posture 3, 166–169 (1995).
Ugbolue, U. C. et al. The evaluation of an inexpensive, 2D, video based gait assessment system for clinical use. Gait Posture 38, 483–489 (2013).
Wong, W. Y. & Wong, M. S. Trunk posture monitoring with inertial sensors. Eur. Spine J. 17, 743–753 (2008).
Ylinen, J., Nykänen, M., Kautiainen, H. & Häkkinen, A. Evaluation of repeatability of pressure algometry on the neck muscles for clinical use. Man Ther. 12, 192–197 (2007).
Walton, D. M. et al. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J. Orthop. Sports Phys. Ther. 41, 644–650 (2011).
Koppenhaver, S. L. et al. Changes in lumbar multifidus muscle function and nociceptive sensitivity in low back pain patient responders versus non-responders after dry needling treatment. Man Ther. 20, 769–776 (2015).
Balaguier, R., Madeleine, P. & Vuillerme, N. Is one trial sufficient to obtain excellent pressure pain threshold reliability in the low back of asymptomatic individuals? A test–retest study. PLoS One. 11, e0160866 (2016).
Castien, R. F., van der Wouden, J. C. & De Hertogh, W. Pressure pain thresholds over the cranio-cervical region in headache: A systematic review and meta-analysis. J. Headache Pain. 19, 9 (2018).
Martínez-Segura, R., De-la-Llave-Rincón, A. I., Ortega-Santiago, R., Cleland, J. A. & Fernández-de-Las-Peñas, C. Immediate changes in widespread pressure pain sensitivity, neck pain, and cervical range of motion after cervical or thoracic thrust manipulation in patients with bilateral chronic mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 42, 806–814 (2012).
Balaguier, R., Madeleine, P. & Vuillerme, N. Intra-session absolute and relative reliability of pressure pain thresholds in the low back region of vine-workers: Effect of the number of trials. BMC Musculoskelet. Disord. 17, 350 (2016).
Neblett, R., Hartzell, M. M., Mayer, T. G., Cohen, H. & Gatchel, R. J. Establishing clinically relevant severity levels for the central sensitization inventory. Pain Pract. 17, 166–175 (2017).
Kregel, J. et al. The Dutch central sensitization inventory (CSI): Factor analysis, discriminative power, and test–retest reliability. Clin. J. Pain. 32, 624–630 (2016).
Scerbo, T. et al. Measurement properties of the central sensitization inventory: A systematic review. Pain Pract. 18, 544–554 (2018).
Belgian Headache Society. https://www.belgianheadachesociety.be/bhs/download/headache-calendar-NL.pdf (2020).
Boonstra, A. M. et al. Cut-off points for mild, moderate, and severe pain on the numeric rating scale for pain in patients with chronic musculoskeletal pain: Variability and influence of sex and catastrophizing. Front. Psychol. 7, 1466 (2016).
Cleland, J. A., Childs, J. D. & Whitman, J. M. Psychometric properties of the neck disability index and numeric pain rating scale in patients with mechanical neck pain. Arch. Phys. Med. Rehabil. 89, 69–74 (2008).
Pool, J. J., Ostelo, R. W. & Hoving, J. L. Minimal clinically important change of the Neck Disability Index and the Numerical Rating Scale for patients with neck pain. Spine 32, 3047–3051 (2007).
Young, I. A., Dunning, J., Butts, R., Cleland, J. A. & Fernández-de-Las-Peñas, C. Psychometric properties of the Numeric Pain Rating Scale and Neck Disability Index in patients with cervicogenic headache. Cephalalgia 39, 44–51 (2019).
Mollayeva, T. et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 25, 52–73 (2016).
Marinus, J., Visser, M., van Hilten, J. J., Lammers, G. J. & Stiggelbout, A. M. Assessment of sleep and sleepiness in Parkinson disease. Sleep 26, 1049–1054 (2003).
Buysse, D. J., Reynolds, C. F. 3rd., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989).
Smyth, C. A. Evaluating sleep quality in older adults: The Pittsburgh Sleep Quality Index can be used to detect sleep disturbances or deficits. Am. J. Nurs. 108, 42–50 (2008).
de Beurs, E., Van Dyck, R., Marquenie, L. A., Lange, A. & Blonk, R. D. DASS: Een vragenlijst voor het meten van depressie, angst en stress. Gedragstherapie 34, 35–53 (2001).
Parkitny, L. et al. Rasch analysis supports the use of the depression, anxiety, and stress scales to measure mood in groups but not in individuals with chronic low back pain. J. Clin. Epidemiol. 65, 189–198 (2012).
Lovibond, P. F. & Lovibond, S. H. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 33, 335–343 (1995).
Buse, D. C. et al. Why HURT? A review of clinical instruments for headache management. Curr. Pain Headache Rep. 16, 237–254 (2012).
Martin, M., Blaisdell, B., Kwong, J. W. & Bjorner, J. B. The Short-Form Headache Impact Test (HIT-6) was psychometrically equivalent in nine languages. J. Clin. Epidemiol. 57, 1271–1278 (2004).
Kosinski, M. et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 12, 963–974 (2003).
Kawata, A. K. et al. Psychometric properties of the HIT-6 among patients in a headache-specialty practice. Headache 45, 638–643 (2005).
Alburquerque-Sendín, F., Madeleine, P., Fernández-de-Las-Peñas, C., Camargo, P. R. & Salvini, T. F. Spotlight on topographical pressure pain sensitivity maps: A review. J. Pain Res. 11, 215–225 (2018).
O’Sullivan, P. et al. Sensory characteristics of chronic non-specific low back pain: A subgroup investigation. Man Ther. 19, 311–318 (2014).
Prushansky, T., Dvir, Z. & Defrin-Assa, R. Reproducibility indices applied to cervical pressure pain threshold measurements in healthy subjects. Clin. J. Pain. 20, 341–347 (2004).
Finocchietti, S., Nielsen, M., Mørch, C. D., Arendt-Nielsen, L. & Graven-Nielsen, T. Pressure-induced muscle pain and tissue biomechanics: A computational and experimental study. Eur. J. Pain. 15, 36–44 (2011).
Chesterton, L. S., Sim, J., Wright, C. C. & Foster, N. E. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin. J. Pain. 23, 760–766 (2007).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (2nd edn.) 579 (Routledge, 1988).
Hodges, P. W., Coppieters, M. W., MacDonald, D. & Cholewicki, J. New insight into motor adaptation to pain revealed by a combination of modelling and empirical approaches. Eur. J. Pain. 17, 1138–1146 (2013).
Reed, C. L. et al. Body matters in emotion: Restricted body movement and posture affect expression and recognition of status-related emotions. Front. Psychol. 11, 1961 (2020).
Bartlett, R., Wheat, J. & Robins, M. Is movement variability important for sports biomechanists?. Sports Biomech. 6, 224–243 (2007).
Diniz, A. et al. Contemporary theories of 1/f noise in motor control. Hum. Mov. Sci. 30, 889–905 (2011).
Latash, M. L., Scholz, J. P. & Schröner, G. Motor control strategies revealed in the structure of motor variability. Exerc. Sport Sci. Rev. 30, 26–31 (2002).
Davids, K., Bennett, S. & Newell, K. Movement System Variability. (eds. Davids, K., Bennett, S. & Newell, K) 49–72 (Human Kinetics, 2005).
Hagg, G. Static work loads and occupational myalgia—A new explanation model. In Electromyographical Kinesiology, Proceedings of the 8th Congress of the International Society of Electrophysiological Kinesiology, Baltimore, Maryland, USA, 12–16 August 1990, 75–78 (Pergamon Press, 1990).
Hamill, J., Palmer, C. & Van Emmerik, R. E. Coordinative variability and overuse injury. Sports Med. Arthrosc. Rehabil. Ther. Technol. 4, 45 (2012).
Bernstein, N. The Coordination and Regulation of Movement. 196 (Pergamon Press, 1967).
Cote, J. N., Feldman, A. G., Mathieu, P. A. & Levin, M. F. Effects of fatigue on intermuscular coordination during repetitive hammering. Mot. Control 12, 79–92 (2008).
Madeleine, P. & Farina, D. Time to task failure in shoulder elevation is associated to increase in amplitude and to spatial heterogeneity of upper trapezius mechanomyographic signals. Eur. J. Appl. Physiol. 102, 325–333 (2008).
Szeto, G. P., Straker, L. M. & O’Sullivan, P. B. A comparison of symptomatic and asymptomatic office workers performing monotonous keyboard work–1: Neck and shoulder muscle recruitment patterns. Man Ther. 10, 270–280 (2005).
Pooriput, W., Bala, R. & Prawit, J. Internal oblique and transversus abdominis muscle fatigue induced by slumped sitting posture after 1 hour of sitting in office workers. Saf. Health Work 7, 49–54 (2016).
Black, K. M., McClure, P. & Polansky, M. The influence of different sitting positions on cervical and lumbar posture. Spine 21, 65–70 (1996).
Patwardhan, A. G. et al. Postural consequences of cervical sagittal imbalance: A novel laboratory model. Spine 40, 783–792 (2015).
Mingels, S., Dankaerts, W. & Granitzer, M. Is there support for the paradigm “spinal posture as a trigger for episodic headache”? A comprehensive review. Curr. Pain Headache Rep. 23, 17 (2019).
National Center for Biotechnology Information. PubMed https://www.ncbi.nlm.nih.gov/mesh/68006706 (2020).
Côté, J. N. A critical review on physical factors and functional characteristics that may explain a sex/gender difference in work-related neck/shoulder disorders. Ergonomics 55, 173–182 (2012).
Svendsen, J. H. & Madeleine, P. Amount and structure of force variability during short, ramp and sustained contractions in males and females. Hum. Mov. Sci. 29, 35–47 (2010).
Gaudart, C. Conditions for maintaining ageing operators at work—A case study conducted at an automobile manufacturing plant. Appl. Ergon. 31, 453–462 (2000).
van Dieën, J. H., Westebring-van der Putten, E. P., Kingma, I. & de Looze, M. P. Low-level activity of the trunk extensor muscles causes electromyographic manifestations of fatigue in absence of decreased oxygenation. J. Electromyogr. Kinesiol. 19, 398–406 (2009).
Madeleine, P. On functional motor adaptations: From the quantification of motor strategies to the prevention of musculoskeletal disorders in the neck–shoulder region. Acta Physiol. 199, 1–46 (2010).
Georgoulis, A. D., Moraiti, C., Ristanis, S. & Stergiou, N. A novel approach to measure variability in the anterior cruciate ligament deficient knee during walking: the use of the approximate entropy in orthopaedics. J. Clin. Monit. Comput. 20, 11–18 (2006).
Jeng, S. F., Holt, K. G., Fetters, L. & Certo, C. Self-optimization of walking in nondisabled children and children with spastic hemiplegic cerebral palsy. J. Mot. Behav. 28, 15–27 (1996).
van den Hoorn, W., Bruijn, S. M., Meijer, O. G., Hodges, P. W. & van Dieën, J. H. Mechanical coupling between transverse plane pelvis and thorax rotations during gait is higher in people with low back pain. J. Biomech. 45, 342–347 (2012).
Srinivasan, D., Rudolfsson, T. & Mathiassen, S. E. Between- and within-subject variance of motor variability metrics in females performing repetitive upper-extremity precision work. J. Electromyogr. Kinesiol. 25, 121–129 (2015).
Fillingim, R. B. Individual differences in pain: Understanding the mosaic that makes pain personal. Pain 158, S11–S18 (2017).
Madden, V. J. et al. Variability in experimental pain studies: Nuisance or opportunity?. Br. J. Anaesth. 126, e61–e64 (2021).
Lomond, K. V. & Cote, J. N. Movement timing and reach to reach variability during a repetitive reaching task in persons with chronic neck/shoulder pain and healthy subjects. Exp. Brain Res. 206, 271–282 (2010).
Winter, D. A. Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological. 143 (University of Waterloo Press, 1991).
Madeleine, P., Nielsen, M. & Arendt-Nielsen, L. Characterization of postural control deficit in whiplash patients by means of linear and nonlinear analyses—A pilot study. J Electromyogr. Kinesiol. 21, 291–297 (2011).
Stergiou, N. & Decker, L. M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection?. Hum. Mov. Sci. 30, 869–888 (2011).
Stergiou, N., Harbourne, R. & Cavanaugh, J. Optimal movement variability: A new theoretical perspective for neurologic physical therapy. J. Neurol. Phys. Ther. 30, 120–129 (2006).
Acknowledgements
The authors want to acknowledge Odekerken D., Diederen T. and Koppert M. for their technical support throughout the study.
Funding
This work was supported by the Special Research Fund (BOF) of Hasselt University (BOF19OWB28).
Author information
Authors and Affiliations
Contributions
S.M., W.D., L.V.E., L.B. and M.G. conceived and planned the experiments. S.M. carried out the experiments and took the lead in writing the manuscript. W.D., L.V.E. and M.G. supervised and provided feedback throughout the entire research and writing phase. L.B. supported the data-analysis and provided statistical support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mingels, S., Dankaerts, W., van Etten, L. et al. Spinal postural variability relates to biopsychosocial variables in patients with cervicogenic headache. Sci Rep 11, 13783 (2021). https://doi.org/10.1038/s41598-021-93138-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93138-3
- Springer Nature Limited