Abstract
Gastroenteritis is common among children. Campylobacter jejuni is one of the main causative bacterial pathogens, together with Shigella, Salmonella and invasive Escherichia coli. Campylobacteriosis is a zoonotic, usually self-limited disease that does not always require antibiotic treatment. In cases of protracted diarrhoea in healthy children or immunocompromised patients, antibiotic treatment is recommended, and the drug of choice is still macrolides, with very low resistance rates in Campylobacter species. However, it is crucial to isolate the causative organism, because some cases, such as Shigella encephalitis, call for initiation of empiric antibiotic treatment. In this study, we compared the incidence, epidemiology, clinical findings and laboratory results of gastroenteritis with dysentery caused by these organisms in children in our area. C. jejuni was found to be the leading pathogen in children hospitalized with bacterial gastroenteritis, followed by Shigella and Salmonella. Macrolides were the drug of choice for Campylobacter, and ceftriaxone and ciprofloxacin were the best empiric treatments for Shigella and Salmonella, respectively.
Similar content being viewed by others
Introduction
Acute bacterial gastroenteritis is a common disease in infants and children. The clinical presentation includes fever, diarrhoea, bloody stool, vomiting, dehydration and abdominal pain.
Campylobacter species, Shigella, Salmonella and invasive Escherichia coli are the main bacterial organisms causing acute infection in the gastrointestinal tract1. Campylobacter organisms are thin curved Gram-negative non-spore-forming rods. The Campylobacter group includes 26 species: the most frequent types that cause disease in humans are Campylobacter jejuni, Campylobacter coli and Campylobacter fetus2, these are also the most common types in Israel3.
Campylobacteriosis is a zoonotic disease, with wild birds, poultry and domestic pets as the main reservoirs. Outbreaks are often linked to contaminated water, unpasteurized milk products, consumption of under-cooked chicken, environmental exposure and contact with farm animals4. It is difficult to differentiate acute gastroenteritis caused by Campylobacter from gastroenteritis caused by Shigella or Salmonella based only on clinical signs or routine blood analysis; stool culture provides the definitive diagnosis of the causative organism. In cases of severe dehydration, toxic appearance, signs of sepsis and gastroenteritis caused by Shigella or Salmonella, initiation of empiric antibiotic treatment, such as third-generation cephalosporin, is required; in cases suspected of Shigella encephalitis, empiric treatment is also recommended. Third-generation cephalosporin has no effect against Campylobacter4.
Campylobacter is considered the causative organism in most cases of bacterial gastroenteritis in young children. It is usually a self-limited disease and does not always require antibiotic treatment. In cases of protracted diarrhoea in healthy children, or infection in immunocompromised patients, antibiotic treatment is recommended, and the drug of choice is still macrolide antibiotics, with very low resistance rates in Campylobacter species5.
The goals of the present study were to examine whether C. jejuni is the leading pathogen of acute gastroenteritis in children admitted with diarrhoea and bloody stool to paediatric departments, and to compare the demographic data, clinical findings and routine blood analysis results to the same data in patients presenting with other pathogens that cause a similar disease.
Methods
We searched for children who were hospitalized between January 2003 and December 2012 at our medical centre in the north of Israel, which serves a population of Jewish, and Muslim and Christian Arab origins. The patients were admitted to the paediatric department with main complaints of acute gastroenteritis, bloody stools or signs of dysentery. We included only patients with positive stool cultures. Routine policy is to obtain blood cultures from patients who suffer from fever during presentation or hospitalization. We analysed demographic data, and clinical signs, such as fever, vomiting and dehydration. We compared these data from cases with positive stool culture for Campylobacter spp. to those with other pathogens that cause bloody diarrhoea in the same cohort. At our centre, we do not perform antibiograms for Campylobacter that causes acute gastroenteritis. For the other organisms, we test for sensitivity patterns to various antibiotics. We explored other complications, such as bacteraemia, and we also looked for the treatment received by the patient. The purpose of our study was to compare the incidence of Campylobacter as a cause of bloody diarrhoea with that of other groups of bacteria that cause a similar disease.
The stool culture was performed as follows. A bean-sized stool specimen was suspended in 2 ml of a 0.9% NaCl solution and used to inoculate one half of a Salmonella Shigella (SS) agar plate and a Campylobacter selective agar plate. The saline stool suspension was then overlaid with Mueller Kaufmann brilliant green bile enrichment broth. Campylobacter plates were incubated in a microaerophilic atmosphere in a jar at 42 °C, while the SS plates were incubated overnight at 37 °C. On the following day, the second half of each SS plate was inoculated with a loop full of the appropriate specimen from the surface of the Mueller Kaufmann brilliant green broth. Suspected Shigella/Salmonella colonies were picked and identified using Kligler enteric test tubes, mass spectrometry (Bruker), and antiserum agglutination. Each culture was incubated for a total of 48 h. Campylobacter plates were evaluated after 24, 48 and 72 h of incubation. Identification was performed using Gram stain according to the Bruker Mass Spectrometry Clinical Microbiology Procedure Handbook (Lynne S. Garcia et al., 2007 update).
All experiments were performed in accordance with the relevant guidelines and regulations. The study received approval from the Helsinki Committee of Emek Medical Center, approval number: EMC-0143-12, issued on 6 Jan 2013. The committee waived the need for informed consent as part of the study approval, since this was a retrospective data analysis.
To analyze the differences between patient characteristics in the different bacterial groups, a series of χ2 tests or Fisher's exact tests (performed when the assumptions of the parametric χ2 test were not met) were conducted. To test whether the groups differed in continuous characteristics data, a linear model ANOVA or nonparametric Kruskal–Wallis rank-sum test (where the sample means did not satisfy the normality assumption) was conducted. The Bonferroni correction was used for multiple comparisons. We computed the two-tailed P values, where P < 0.05 was considered a statistically significant result. Statistical analyses were performed using R statistical software version 3.6.1.
Results
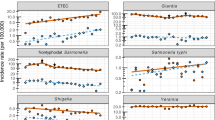
Positive stool cultures were found in 622 patients, representing 18% of all patients hospitalized under the presumptive diagnoses listed in Methods, during the designated time period. Demographic characteristics of the patients according to the three common enteropathogens are presented in Table 1. Overall, 356 (57%) patients were male, 266 (43%) female, and 367 (59%) were of Arab origin. The ethnic distribution in the paediatric population served by our medical centre is 64% Arab (Muslims and Christians) and 35% Jewish. We found significant differences in age between the Campylobacter and Shigella groups and between the Shigella and Salmonella groups (P < 0.001 for both), and no significant difference between the Campylobacter and Salmonella groups (Table 1).
Three groups of bacteria were found in the present study: Campylobacter was isolated from 53.3% of the samples (Table 1). Of these, 288 (86.74%) were C. jejuni isolates, 15 (4.51%) C. coli isolates, and 29 (8.37%) Campylobacter species isolates (our laboratory does not specify the isolates beyond those mentioned). The second group included patients with Shigella; of the isolates found, 145 (91.7%) were Shigella sonnei, followed by 8 (5.7%) S. flexneri and 4 (2.5%) S. boiydee isolates. The Salmonella group included 21.2% of the patients; all of the isolates were Salmonella enterica.
Fever at admission, length of hospital stay and duration of diarrhoea are shown in Table 2, and significant differences were found between groups (Table 2). A comparison of mean temperatures between groups showed a significant difference in fever extent between Campylobacter and Shigella groups (P = 0.003). The average length of hospitalization also showed significant differences between groups (Table 2).
Mean duration of diarrhoea was significantly different between groups (Table 2). We searched for the presence of diarrhoea and bloody diarrhoea in the three groups, but there was no significant difference between the groups. In the Campylobacter, Shigella and Salmonella groups, 67%, 88% and 83% of the patients, respectively, received intravenous fluids.
A total of 1320 blood cultures were obtained during the research period from patients included in the study. Four patients were diagnosed with uncomplicated bacteraemia: S. enterica and two isolations of C. jejuni. In one patient, Staphylococcus coagulase negative was isolated and, in another patient, a Bacillus species was isolated; these two pathogens were considered to be contaminants.
At our institution, we do not perform antibiograms for Campylobacter isolated from stool. The Shigella isolates were sensitive to ciprofloxacin, ceftriaxone and nalidixic acid (Table 3). The Salmonella organisms were sensitive to ceftriaxone and ciprofloxacin (Table 3). The Salmonella isolates were not significantly more sensitive to those antibiotics (P < 0.001 for both drugs) than the Shigella isolates (Table 3)
A total of 267 patients (43%) received antibiotic therapy before and/or after receiving the stool culture results; 87 patients were treated prior to the culture results, and 31 following the culture results. Some data were not available in the medical charts of some patients in the three group, as demonstrated in Table 4. In the Campylobacter, Shigella and Salmonella groups, 40%, 49% and 44% of the patients received antibiotic treatment. In the Campylobacter group, 60% of the patients received macrolides either before or after the culture results; 71% received ceftriaxone, which has no effect against Campylobacter; 19% were treated with a dual empiric antibiotic regimen that included azithromycin and ceftriaxone. Ceftriaxone was mostly used as the empiric treatment in severe cases, and only one patient in the Campylobacter group received ceftriaxone after the culture results arrived. The standard course of ceftriaxone treatment was a single dose per day for 3 days. In the Shigella and Salmonella groups, 82% and 83% of the patients, respectively, received ceftriaxone. Most patients received antibiotics prior to the culture results. As shown in Table 4, there was no significant difference between the number of treatment days before and after culture results among the three groups.
A significant difference was found in the length of the hospital stay (P < 0.001) and diarrhoea duration (P = 0.003) in patients who were treated with antibiotics compared to those who were not treated. These parameters were higher in the patients that were treated (Table 5).
Discussion
Campylobacter species are an important cause of bacterial gastroenteritis, especially among hospitalized children, followed by Shigella and Salmonella. These pathogens should be considered in children who suffer from sudden onset of protracted diarrhoea or have symptoms that appear more toxic than a state of dehydration. Systemic, extra-intestinal dissemination of these pathogens is uncommon, with the exception of Salmonella infection in immunocompromised hosts or during the first year of life. Complications such as bacteraemia are not common in Campylobacter or Shigella infections, but have been described in infants with Salmonella, especially Salmonella typhi. These pathogens may cause neurological complications such as: seizures in Shigella and meningitis in Salmonella and Campylobacter1,2,4,5. Salmonella may also lead to pneumonia and myocarditis. Campylobacter may cause urinary tract infections and cholecystitis6.
As shown here and by others1,4,5,7, Shigella is more common in older children and adults, whereas Campylobacter and Salmonella are more frequently found in younger children and infants. C. jejuni is the most frequently isolated Campylobacter species in cases of gastroenteritis, and it is the most common organism causing foodborne illnesses in the United States.
Generally, Campylobacter infections are mild, mostly self-limited and require only supportive care. Nevertheless, some cases may lead to severe dehydration. Severe cases of protracted diarrhoea and infection in immunocompromised children and young infants may benefit from antibiotic treatment4,5,6,7. Our study did not show a shortened duration of hospitalization or diarrhoea among patients that received antibiotics, possibly due to a more severe course of disease among patients whose condition necessitated antibiotic therapy.
The drugs of choice were found to be ceftriaxone and azithromycin, either alone or as a dual therapy. Ceftriaxone was used mostly as an empiric therapy in severe cases, whereas azithromycin was used as empiric and specific therapy following culture results. We do not perform antibiograms for Campylobacter stool isolates. As mentioned in previous reports, less than 5% of Campylobacter spp. are resistant to macrolides5,7,8. Nonetheless, in cases of serious infection, where there is no response to macrolide treatment, drug sensitivity studies should be performed. Recent studies conducted in Asia and the United States reported increasing rates of Campylobacter resistance to macrolides as well as fluoroquinolones7. The sensitivity of Shigella and Salmonella isolates to ciprofloxacin and ceftriaxone, was not significant in our study. It is important to mention that, ciprofloxacin is not the drug of choice for children due to arthropathy.
Complications caused by Campylobacter gastroenteritis are rare, although early- and late-onset complications have been described. Early-onset complications include: septic arthritis, bursitis, osteitis, soft tissue infections, erythema nodosum, glomerulonephritis, haemolytic anaemia, IgA nephropathy, post-infectious irritable bowel syndrome, and intestinal perforation. Campylobacter is also the most commonly identified cause of Guillain-Barré syndrome and can cause reactive arthritis as a late-onset complication6.
Campylobacter can also cause invasive diseases, including bacteraemia. We found two cases of bacteraemia due to Campylobacter. Very few previous studies have described Campylobacter bacteraemia in Israel or elsewhere9,10,11. Bacteraemia is a rare finding of Campylobacter gastroenteritis in immunocompetent children, but it has been found in several cases of immunocompromised children. The outcome is usually favourable, with the administration of broad-spectrum antibiotic therapy, such as carbapenems, gentamicin or amikacin.
Studies conducted in Peru12 Pakistan13 and Thailand14 have demonstrated results similar to those found here. In those areas, Campylobacter was the major cause of dysentery among children under the age of 5 years, followed by Shigella and other enteropathogens. In our study, campylobacteriosis was found to be more common among infants and young children, and Shigellosis was seen in older children. Campylobacter isolates were sensitive to macrolides, whereas Shigella isolates were sensitive to third-generation cephalosporin and quinolones.
In conclusion, C. jejuni is one of the most common causes of bacterial gastroenteritis in young hospitalized children, and is usually self-limited. Severe cases should be treated with antibiotics, usually macrolides, although increasing rates of resistance, especially to macrolides and fluoroquinolones, should be considered. Even though complications are rare, it is important to isolate the causative organism from the stool, to initiate the appropriate therapy.
Ethical approval
This study received approval from the Helsinki Committee of the Emek Medical Center, approval number: EMC-0143-12, issued on 6 Jan 2013.
Data availability
All data generated or analysed during this study are included in this published article.
References
Amieva, M. R. Important bacteria gastrointestinal pathogens in children: a pathogenesis perspective. Pediatr. Clin. North Am. 52, 749–777 (2005).
Fittzgerald, C. Campylobacter. Clin. Lab. Med. 5, 289–298 (2015).
Weinberger, M. Increased incidence of Campylobacter spp. iinfection and high rates among children, Israel. Emerging Infectious Diseases 19, 1828–31 (2013).
Domingues, A. R., Pires, S. M., Halasa, T. & Hald, T. Source attribution of human Campylobacteriosis using meta-analysis of case-control studies of sporadic infections. Epidemiol. Infect. 140, 970–981 (2012).
Stutman, H. R. Salmonella, Shigella and Campylobacter: common bacterial causes of infectious diarrhea. Pediatr. Ann. 23(10), 538–543 (1994).
Gupta, A., Nelson, J. M. & Barrett, T. J. Antimicrobial resistance among Campylobacter strains, United States, 1997–2001. Emerg. Infect. Dis. 10, 1102–1109 (2004).
Same, R. G. & Tamma, P. D. Campylobacter infections in children. Pediatr. Rev. 39, 533–541 (2018).
Bolinger, H. & Kathariou, S. The current state of macrolide resistance in Campylobacter spp.: trends and impacts of resistance mechanisms. Appl. Environ. Microbiol. 83, 1–9 (2017).
Pham, N. T. et al. Antibiotic resistance of Campylobacter jejuni and C. coli isolated from children with diarrhea in Thailand and Japan. Jpn. J. Infect. Dis. 69, 77–79 (2016).
Ben-Shimol, S., Carmi, A. & Greenberg, D. Demographic and clinical characteristics of Campylobacter bacteremia in children with and without predisposing factors. Pediatr. Infect. Dis. J. 32, 414–418 (2016).
Hussein, K. et al. Campylobacter bacteraemia: 16 years of experience in a single centre. Infect. Dis. 48(11–12), 796–799 (2016).
Francois, R. et al. The other Campylobacters: not innocent bystanders in endemic diarrhea and dysentery in children in low-income settings. PLoS Negl. Trop. Dis. 12(2), e0006200 (2018).
Soofi, S. B. et al. A comparison of disease caused by Shigella and Campylobacter species: 24 months community-based surveillance in 4 slums of Karachi, Pakistan. J. Infect. Public Health. 4(1), 12–21 (2011).
Bodhidatta, L. et al. Bacterial enteric pathogens in children with acute dysentery in Thailand: increasing importance of quinolone-resistant. Campylobacter. Southeast Asian J. Trop. Med. Public Health 33(4), 752–7 (2002).
Acknowledgements
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Author information
Authors and Affiliations
Contributions
W.S. contributed to concept and design, acquisition, analysis and interpretation of the data, revising the article, and final approval of the version for publication. Z.H.E. contributed to analysis and interpretation of the data, drafting the article, and final approval of the version for publication. R.S., H.B., R.H. and A.K. contributed to concept and design, revising the article for critically important intellectual content, and final approval of the version for publication. All authors reviewed and agreed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sakran, W., Hexner-Erlichman, Z., Spiegel, R. et al. Campylobacter gastroenteritis in children in north-eastern Israel comparison with other common pathogens. Sci Rep 10, 5823 (2020). https://doi.org/10.1038/s41598-020-62744-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62744-y
- Springer Nature Limited