Abstract
Extreme heat in summer is frequent in parts of China, and this likely affects the fitness of the beetle Ophraella communa, a biological control agent of invasive common ragweed. Here, we assessed the life history parameters of O. communa when its different developmental stages were exposed to high temperatures (40, 42 and 44 °C, with 28 °C as a control) for 3 h each day for 3, 5, 5, and 5 days, respectively (by stage). The larval stage was the most sensitive stage, with the lowest survival rate under heat stress. Egg and pupal survival significantly decreased only at 44 °C, and these two stages showed relative heat tolerance, while the adult stage was the most tolerant stage, with the highest survival rates. High temperatures showed positive effects on the female proportion, but there was no stage-specific response. Treated adults showed the highest fecundity under heat stress and a similar adult lifespan to that in the control. High temperatures decreased the F1 egg hatching rate, but the differences among stages were not significant. Negative carry-over effects of heat stress on subsequent stages and progenies’ survival were also observed. Overall, heat effects depend on the temperature and life stage, and the adult stage was the most tolerant stage. Ophraella communa possesses a degree of heat tolerance that allows it to survive on hot days in summer.
Similar content being viewed by others
Introduction
Common ragweed, Ambrosia artemisiifolia, an invasive weed, is a nationwide problem with ecological and health costs in China1. It is usually found alongside roads and in crop fields and orchards2. It has gradually spread and can now be found in 21 provinces in China1,3. Ophraella communa originates from North America4; accordingly, in China, it is used as a biological control agent of A. artemisiifolia1, with both larvae and adults feeding on A. artemisiifolia leaves1. When the beetles occur at a high population size, they exert strong control of the invasive weed5,6, and they significantly reduce seed production, even if a few defoliated plants survive7. With the rapid expansion, broad dispersal, high productivity, high feeding amount8, and rapid evolution of O. communa9, the beetle has provided complete defoliation and prevented flowering and seed set in ragweed plants in Europe10. In recent years, this natural enemy has been reported in eastern11, central12, and southern parts of China13, and it has been shown to provide effective control of A. artemisiifolia in the field in China at some sites1.
Research on O. communa has provided important insights into temperature. Temperature is a dominant abiotic factor that strongly affects organisms’ behaviour, physiology, life history, distribution, and abundance14. Insects have an optimal temperature range to which their biological functions are best adapted; under supra-optimal temperatures, insects might incur physiological costs and suffer damage that lowers their performance15. Most insects have the ability to tolerate some degree of temperature fluctuation16, but lethal temperatures are usually between 40 and 50 °C depending on insect species and life stage17. Extreme heat in summer has become more frequent in recent years compared to in the early 20th century in many regions around the world18. In many parts of China, summer maximum daily temperatures in the field often exceed 40 °C for several hours, and the number of such hot days has also increased in the last few years19,20,21. Heat shock affects the developmental fitness and behaviour of insects22. The effects of heat stress have been reported in a variety of insects, including Trialeurodes vaporariorum31, any developmental stage of O. communa might encounter a brief period of high-temperature stress; therefore, the developmental fitness of this beetle may be adversely affected when heat-sensitive life stages experience summer heat. Overall, our findings reflect that the intensity of the high temperature, the developmental stage, and their interaction all had significant influences on the life history parameters of O. communa.
Survival under heat stress
In response to high temperatures, insects may die rapidly due to serious heat injury27,33,52, and a decrease in female fertility caused by heat shock is likewise described in T. euproctidis45. On the other hand, heat stress greatly decreased the frequency of courtship and mating by reducing the attractiveness of males to females in three Drosophila species (D. melanogaster, D. simulans and D. mojavensis)55 and the diamondback moth, P. xylostella49. It is reported that O. communa females laid fewer eggs with short mating time, and the copulation time was decreased with increasing temperature56. Multiple mating (female acceptance of copulations with different males (polyandry) or repeated copulations with the same male (monogamy)) has a positive effect on egg production has been reported in many insects57,58,59. Ophraella communa adults mate many times throughout their lifespan and even mate several times in one day, its fitness parameters are positively associated with the number of copulation events, and multiple-mating behaviour increase the fitness benefits60. Adult females of O. communa may mate with multiple males in a lifetime in the field due to their strong activity, our methods (repeated copulations with the same male) may underestimate the real reproduction of O. communa. Meanwhile, the replacement of earlier died males with other males of the similar age and treatment will change the numbers of mated males, which will influence the results. We also suggests that the effect of mating patterns under heat stress to address in future. The mechanisms of the decrease in reproductive output after temperature stress may be due to impaired oocyte development, decreased mating success, sperm production, sperm viability61, and changed mating patterns58.
Heat stress effects on lifespan
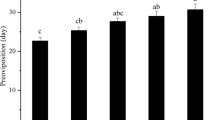
In general, a straightforward trade-off between damage repair and somatic maintenance could reduce longevity61. Our previous study showed that the longevity of O. communa adults significantly decreased after the exposure of adults to high temperatures ≥35 °C for 2 h3, which was consistent with this general principle. However, overall, heat stress had no significant effect on the lifespan of adult O. communa after the exposure of adults to high temperatures ≥40 °C for 3 h in the present study, which differed from the previous results. Mild temperature hardening in nature can increase insects’ thermotolerance51; thus, the time at which insect samples are collected from the field also influences adult longevity under heat stress. Some previous studies also reported that heat stress increased the longevity of some Drosophila species62, such as D. melanogaster males (exposure of adults to 34 °C for 3 h)63, parasitoids, such as females of the wasp A. avenae (exposure of adults to 36 °C for 1 h)35, and the oriental fruit moth, Grapholita molesta (exposure of adults to 38 °C for 4 h)64. In our study, there was no obvious change trend in adult O. communa longevity after exposure of the preadult stages to high temperatures ≥40 °C for 3 h, and an increase or decrease may be caused by chance. Different methods of heat exposure may have different levels of among-stages and species-related variance. Fluctuating high temperatures provided the chance for surviving insects to improve their heat tolerance and fitness, including longevity65,66. Thus, the lifespan of O. communa may be longer in the field in summer heat than that under constant high-temperature conditions in the lab.
Heat stress effects on progeny
The fitness of offspring might also be affected by high maternal temperatures49. The F1 egg hatching of O. communa was likewise directly proportional to the high temperatures in our study, which indicated that the effects of heat shock could be transferred to the next generation27. Previous studies found that F1 egg-hatching rates were affected by high temperature in the whitefly T. vaporariorum23 and the fruit flies D. melanogaster67 and S. crassipalpis52. Extreme examples showed that heat stress affected T. euproctidis and S. crassipalpis males, and this resulted in no eggs being fertilised45,52. Male sterility or reduced fertility caused by heat stress was likewise found in D. buzzatii, and this also affected progeny fitness50,68. The decrease in the egg hatching rates in the next generation of O. communa after exposure of different life stages to high temperatures in this study might be due to male infertility.
Stage-specific heat effects
Stage-specific heat tolerance has been observed in many insects14,19,32,52,65. The life history traits of O. communa were also affected by its different developmental stages being exposed to heat stress in our study. Normally, less mobile stages (like eggs and pupae) are more resistant to heat than mobile stages (like larvae and adults)30. By contrast, from the standpoint of survival, in O. communa, the larval stage was the most heat-susceptible life stage, and the adult stage was the least heat-susceptible life stage in our study. The relative sensitivity of less mobile stages was observed in P. xylostella (eggs and pupae)19 and Wyeomyia smithii (pupae)69, and the relative insensitivity of mobile stages was also observed in Tenebrio molitor (adults)70. Stress resistance may be affected by past selection pressures depending on the environments in which the different developmental stages are found19. Plutella xylostella eggs and pupae usually occur on the underside of leaves, where temperatures are cooler on hot days, and this may help explain the relative sensitivity of these stages compared to the pattern in other insects19. Ophraella communa prefers to lay eggs on the back of the mid and basal leaves, and first instar larvae stay for several hours near the egg shell (personal observation), which may contribute to our understanding of the high immediate and subsequent death of eggs and first instar larvae. However, overall, the female proportion and F1 egg hatching rate of O. communa were not significantly affected by the developmental stage exposed. The fecundities and adult longevities of O. communa appeared to be more depressed by heat stress during the pupal stage than during other stages. We assume that adults are more heat tolerant than other stages based on their high survival rate, high female fecundity, longevity that is similar to control adult longevity and relatively high F1 egg hatching rate. Ophraella communa overwinters and expands its distribution mainly in the adult stage9, suggesting that adults are better able to tolerate environmental stress than other stages. Mobile adults likely experience a greater range of thermal microclimates, and greater variability in tolerance or greater basal (innate) tolerance might be expected28. The greater mobility of larvae and adults compared to other stages allows them to search for low-temperature microclimates to reduce thermal injury through behavioural thermoregulation71, which increases the thermal tolerance of larvae and adults in the field. The stage-specific heat tolerance of O. communa is beneficial for the establishment and expansion of this natural enemy in the field.
Potential application in biological control
Temperature is not constant in the field, which varies over time. The prior experience of natural conditions in the field could improve the heat tolerance of some insects16. Ectotherms exposed to daily thermal fluctuations usually showed higher upper thermal limits than those exposed to constant temperature conditions72, which implies that they may be able to survive in the field at higher temperatures than predicted from laboratory experiments conducted under constant temperatures61. The field microclimates experienced by each life stage can be used to inform the avoidance of extreme temperatures70, which will increase insects’ thermal tolerance in the field. Likewise, the results from our constant temperature model might underestimate the thermal tolerance of O. communa in the field. Meanwhile, humidity is also an important abiotic factor influencing the biology of insects. The effects of temperature depend on the relative humidity (RH) level73, and RH also changes with time. Therefore, the life history parameters of O. communa in environments in which thermal and RH environments fluctuate need to be investigated in future studies. Many of the changes in insect development and reproduction may result from changes in the endocrine system22, and insects must constantly adjust their physiologies to changing thermal conditions61. The physiological mechanisms and the secondary sex ratio of O. communa in response to heat stress should be further studied to improve the use of this biological control agent. The results indicate that O. communa can tolerate 44 °C heat for up to 3 hours, which may contribute to its expansion into the lower latitudes in China, where its host (common ragweed) is widely distributed. We conclude that O. communa possesses a degree of heat tolerance that allows it to survive on hot days in summer.
Materials and Methods
Host plants
Ambrosia artemisiifolia seeds were collected from more than ten thousand plants in the town of Da**g (28°56′26″N, 113°14′38″E) in Miluo County, Yueyang City, Hunan Province, China, in late October 201074. The seeds were then stored at 4 °C. Adequately stored seeds were germinated in a greenhouse in late March 2011, and when the seedlings reached a height of approximately 15 cm74, some of them were used in adult heat treatments and tests of longevity, fecundity, and F1 egg hatching in O. communa. The apical buds of the remaining seedlings were removed to prevent apical dominance, and the seedlings were transplanted into pots (21 × 17 cm) containing soil at one seedling per pot. One thousand pots containing treated common ragweed seedlings were prepared and placed in a greenhouse. All the plants were watered in a timely manner and fertilised (N:P:K = 13:7:15) twice per month to maintain normal growth31. The potted plants were used in the heat treatments of eggs, larvae and pupae when the plants were approximately 40 cm high.
Insects
More than 1,000 O. communa adults were collected from the town of Da**g (28°56′26″N, 113°14′38″E) in Miluo County, Yueyang City, Hunan Province, China, on June 24, 2011. Colonies of the beetle were maintained on A. artemisiifolia plants under natural light in a greenhouse at 28 ± 2 °C at the Institute of Plant Protection, Hunan Academy of Agricultural Sciences (25°21′18″N, 114°33′40″E), Changsha, Hunan Province, China74.
Six pairs of O. communa adults were randomly collected from the rearing colony and placed with the aid of a fine brush (size 0) onto a pot containing a fresh common ragweed plant, which was then covered with nylon gauze (40 mesh size). After a 2-d oviposition period, the beetles were removed to synchronise the development of stages for exposure to the thermal treatments. Approximately 400 plants were prepared for the following high-temperature stress treatments.
Thermal treatments
The duration and intensity of heat stress were based on the duration and intensity of high temperatures in summer, which are usually a few hours of particularly high temperatures in central China (max temperature 44 °C for approximately 3 h per day for 3–5 consecutive days)20. The treatments examined the effects of high temperature (40, 42, and 44 °C) on beetle life history parameters using periods of exposure of 3 h per day for 3, 5, 5, and 5 days for eggs, larvae, pupae, and adults, respectively. Control insects were kept at 28 °C to allow normal O. communa development31. The exposure periods were determined based on the developmental periods of the different developmental stages of O. communa (4.0 days for eggs, 7.6 days for larvae, and 6.0 days for pupae) obtained at a constant high temperature (32 °C) in an earlier laboratory bioassay31 and the hottest days (up to 44 °C) that occur for a duration of 3–5 days in Changsha, Hunan Province, China20. The experiments were conducted in early to mid-July 2011 (the field temperature during July in Changsha was 23–40 °C, with an average of 31.5 °C). The high-temperature exposure treatments for each treatment were performed separately in environmental chambers (PRX-450D, Ningbo Haishu Safe Experimental Equipment Co. Ltd., Zhejiang, China) at 28 (untreated control), 40, 42, or 44 ± 1 °C, with a RH of 70 ± 5%. The optimal RH at 25 °C for the development of O. communa in the laboratory ranges from 75% to 90%31. In recent years, the RH in Changsha fluctuated around 70% in summer (personal observation). Therefore, we selected 70% RH as the experimental condition. The exposure treatments were also conducted under a photoperiod of 14:10 (L:D) h31 and a light intensity of 12,000 LX for 3 h daily for 3 or 5 consecutive days.
Effects of high temperatures on survival and female proportion
One hundred eggs ≤12 h old, 90 first instar larvae ≤24 h old, and 55 pupae ≤24 h old were separately retained on three potted plants. Twenty ragweed plants were used for each developmental stage, and they were then exposed to high temperatures in environmental chambers, after which the infested potted plants were kept in a greenhouse. A total of 240 ragweed plants were used. Following the high-temperature stress treatments, the treated pupae were collected by detaching the leaves on which they occurred and placing the individual leaves into open transparent plastic boxes (19 × 12 × 6 cm) in an unsealed plastic cuvette tube covered with nylon gauze (60 mesh size) in the laboratory at 28 ± 2 °C and 70 ± 5% RH, where the pupae were checked daily for adult emergence. The treated eggs and larvae were kept in a greenhouse until they reached the pupal stage. The process for these pupae was the same as that for the treated pupae following the high-temperature stress treatments. The sex of each newly emerged adult was determined using a stereomicroscope, and the female proportion was calculated. The survival (in days) of male and female adults was recorded for each temperature treatment.
Newly emerged adults ≤24 h old (125 pairs) were randomly selected from the rearing colony for exposure to high temperatures. Each adult pair was released onto a fresh ragweed seedling (15 cm height) in a plastic box (19 × 12 × 6 cm) with a hole (15 × 4 cm) covered with nylon gauze (60 mesh size). The survival of male and female adults was checked daily.
The survival rates for eggs, larvae, and pupae were determined using the following equation: (number of emerged individuals of the next stage)/(number individuals in the treated stage) × 100%. The survival rate of adults was determined using the following equation: (number of survived adults)/(number of treated adults) × 100%. The subsequent survival rates of treated eggs and larvae were determined using the following equation: (number of emerged adults)/(number of individuals in the treated stage) × 100%. The female proportion was determined using the following equation: (number of females)/(total number of females and males) × 100%.
Effects of high temperatures on adult longevity, fecundity, and F1 egg hatching
Once the test insects reached the adult stage, insects for which different life stages had been exposed to a range of high temperatures as described above were evaluated for fecundity and adult longevity in the greenhouse. To measure fecundity and longevity, each pair of adults was placed on a fresh potted ragweed seedling in a plastic box (19 × 12 × 6 cm) with a hole (15 × 4 cm) covered with nylon gauze (60 mesh size), with twenty boxes treated as experimental replicates31. The number of eggs laid by the females and the duration of adult survival were recorded daily until all adults died. For each treatment, 1,600 eggs were retained on 20 seedlings to evaluate the egg hatching rate in the greenhouse. Other eggs were removed after counting, and the seedlings were changed when necessary. Egg viability was estimated based on the number of emerged larvae. If a male died, then another treated male of approximately the same age was added (the longevity of these males was not recorded)31.
Statistical analyses
Data were checked for normality and homoscedasticity and, if needed, were arcsine square root or log transformed. All data were analysed using SPSS 21.0 (SPSS Inc., Chicago, Illinois, USA). The survival rate, female proportion, and F1 egg hatching rate (%) were arcsine square root transformed, and adult longevity was transformed using log10 (x + 1) before analysis31. The data were subjected to two-way analysis of variance (ANOVA) to test the effects of temperature, the stage exposed to the heat treatment, and their interaction on the life history parameters of O. communa. Means were separated using Tukey’s HSD (honestly significant difference) test (one-way ANOVA) when significant differences were found at P < 0.05 and were denoted as the means ± SE (standard error of the mean).
References
Zhou, Z. S., Wan, F. H. & Guo J. Y. Common ragweed Ambrosia artemisiifolia L. In Biological Invasions and Its Management in China Volume 2 (eds Wan, F. H., Jiang, M. X. & Zhan, A. B.) 99–109 (Springer, New York, 2017).
Wan, F. H., Hou, Y. M. & Jiang, M. X. Invasion Biology. (Science Press Bei**g, 2015).
Zhou, Z. S., Guo, J. Y., Luo, M. & Wan, F. H. Effect of short-term high temperature stress on the development and fecundity of Ophraella communa (Coleoptera: Chrysomelidae). Biocontrol Sci. Technol. 21, 809–819 (2011).
LeSage, L. A taxonomic monograph of the Nearctic galerucine genus Ophraella Wilcox (Coleoptera: Chrysomelidae). Mem. Entomol. Soc. Can. 118, 3–75 (1986).
Teshler, M. P., Ditommaso, A., Gagnon, J. A. & Watson, A. K. Ambrosia artemisiifolia L., common ragweed (Asteraceae). In Biological Control Programmes in Canada, 1981–2000 (eds Mason, P. G. & Huber, J. T.) 290–294 (CABI Publishing, Wallingford, 2002).
Kiss, L. Why is biocontrol of common ragweed, the most allergenic weed in Eastern Europe, still only a hope? In Biological Control: A global Perspective (eds Vincent, C., Goettel, M. S. & Lazarovits, G.) 80–91 (CABI Publishing, Wallingford, 2007).
Throop, H. L. Nitrogen deposition and herbivory affect biomass production and allocation in an annual plant. Oikos 111, 91–100 (2005).
Bonini, M. et al. Is the recent decrease in airborne Ambrosia, pollen in the Milan area due to the accidental introduction of the ragweed leaf beetle Ophraella communa? Aerobiologia 31, 499–513 (2015).
Tanaka, K., Murata, K. & Matsuura, A. Rapid evolution of an introduced insect Ophraella communa LeSage in new environments: temporal changes and geographical differences in photoperiodic response. Entomol. Sci. 18, 104–112 (2015).
Müller-Schärer, H. et al. Ophraella communa, the ragweed leaf beetle, has successfully landed in Europe: fortunate coincidence or threat? Weed Res. 54, 109–119 (2014).
Meng, L. & Li, B. P. Advances on biology and host specificity of the newly introduced beetle, Ophraella communa Lesage (Coleoptera: Chrysomelidae), attacking Ambrosia artemisiifolia (Compositae) in continent of China. Chinese J. Biol. Cont. 21, 65–69 (2005).
Chen, H. S. et al. A field test of joint control of the alien invasive weed Ambrosia artemisiifolia with Ophraella communa and Epiblema strenuana. Chinese J. Biol. Cont. 29, 362–369 (2013).
Qi, G. J., Gao, Y., Zhong, F., Shao, Y. Y. & Lv, L. H. Control effect of common ragweed Ambrosia artemisiifolia with Ophraella communa in Fogang, Guangdong Province. J. Environ. Entomol. 34, 315–321 (2012).
Blanckenhorn, W. U., Gautier, R., Nick, M., Puniamoorthy, N. & Schäfer, M. A. Stage- and sex-specific heat tolerance in the yellow dung fly Scathophaga stercoraria. J. Therm. Biol. 46, 1–9 (2014).
Jerbi-Elayed, M., Lebdi-Grissa, K., Foray, V., Muratori, F. & Hance, T. Using multiple traits to estimate the effects of heat shock on the fitness of Aphidius colemani. Entomol. Exp. Appl. 155, 18–27 (2015).
Overgaard, J. & Sørensen, J. G. Rapid thermal adaptation during field temperature variations in Drosophila melanogaster. Cryobiology 56, 159–162 (2008).
Terblanche, J. S. et al. Ecologically relevant measures of tolerance to potentially lethal temperatures. J. Exp. Biol. 214, 3713–3725 (2011).
Intergovernmental Panel on Climate Change (IPCC). Summary for policymakers. In Climate Change 2013: the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker, T. F. et al.) 3–29 (Cambridge University Press, Cambridge, 2014).
Zhang, W., Chang, X. Q., Hoffmann, A., Zhang, S. & Ma, C. S. Impact of hot events at different developmental stages of a moth: the closer to adult stage, the less reproductive output. Sci. Rep. 5, 10436 (2015).
Chen, H. S. et al. Effects of high temperature on body size and weight of Ophraella communa. Biocontrol Sci. Technol. 24, 882–890 (2014).
Wang, S. Y., Liang, N. N., Tang, R., Liu, Y. & Liu, T. X. Brief heat stress negatively affects the population fitness and host feeding of Aphelinus asychis (Hymenoptera: Aphelinidae) parasitizing Myzus persicae (Hemiptera: Aphididae). Environ. Entomol. 45, 719–725 (2016).
Neven, L. G. Physiological responses of insects to heat. Postharvest Biol. Technol. 21, 103–111 (2000).
Cui, X. H., Wan, F. H., **e, M. & Liu, T. X. Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. J. Insect Sci. 8, 24 (2008).
Ma, C. S., Hau, B. & Poehling, H. M. Effects of pattern and timing of high temperature exposure on reproduction of the rose grain aphid, Metopolophium dirhodum. Entomol. Exp. Appl. 110, 65–71 (2004).
Solangi, G. S. et al. Effect of high temperatures on survival and longevity of the predator Cryptolaemus montrouzieri Mulsant. Phytoparasitica 41, 213–219 (2013).
Chiu, M. C., Kuo, J. Jr. & Kuo, M. H. Life stage‐dependent effects of experimental heat waves on an insect herbivore. Econ. Entomol. 40, 175–181 (2015).
Cheng, J. X. et al. Effects of heat shock on the Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 110, 1630–1638 (2017).
Terblanche, J. S., Mitchell, K. A., Uys, W., Short, C. & Boardman, L. Thermal limits to survival and activity in two life stages of false codling moth Thaumatotibia leucotreta (Lepidoptera, Tortricidae). Physiol. Entomol. 42, 379–388 (2017).
Zhao, F., Hoffmann, A. A., **ng, K. & Ma, C. S. Life stages of an aphid living under similar thermal conditions differ in thermal performance. J. insect physiol. 99, 1–7 (2017).
Enriquez, T. & Colinet, H. Basal tolerance to heat and cold exposure of the spotted wing drosophila, Drosophila suzukii. PeerJ 5, e3112 (2017).
Zhou, Z. S., Guo, J. Y., Chen, H. S. & Wan, F. H. Effects of temperature on survival, development, longevity, and fecundity of Ophraella communa (Coleoptera: Chrysomelidae), a potential biological control agent against Ambrosia artemisiifolia (Asterales: Asteraceae). Environ. Entomol. 39, 1021–1027 (2010).
Zhang, W., Rudolf, V. H. W. & Ma, C. S. Stage-specific heat effects: timing and duration of heat waves alter demographic rates of a global insect pest. Oecologia 179, 947–957 (2015).
Mironidis, G. K. & Savopoulou-Soultani, M. Effects of heat shock on survival and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) adults. J. Therm. Biol. 35, 59–69 (2010).
**e, Q., Hou, B. H. & Zhang, R. J. Thermal responses of oriental fruit fly (Diptera: Tephritidae) late third instars: mortality, puparial morphology, and adult emergence. J. Econ. Entomol. 101, 736–741 (2008).
Roux, O., Lann, C. L., van Alphen, J. J. M. & van Baaren, J. How does heat shock affect the life history traits of adults and progeny of the aphid parasitoid Aphidius avenae (Hymenoptera: Aphidiidae)? Bull. Entomol. Res. 100, 543–549 (2010).
Niziolek, O. K., Berenbaum, M. R. & DeLucia, E. H. Impact of elevated CO2 and increased temperature on Japanese beetle herbivory. Insect Sci. 20, 513–523 (2013).
Acharya, K. et al. Life history characteristics of Diorhabda carinulata under various temperatures. Environ. Entomol. 42, 564–571 (2013).
Ma, C. S., Wang, L., Zhang, W. & Rudolf, V. H. W. Resolving biological impacts of multiple heat waves: interaction of hot and recovery days. Oikos 127, 622–633 (2018).
Behnaz, G. & Andrew, N. R. The physiological consequences of varied heat exposure events in adult Myzus persicae: a single prolonged exposure compared to repeated shorter exposures. PeerJ 4, e2290 (2016).
Esperk, T., Kjaersgaard, A., Walters, R. J., Berger, D. & Blanckenhorn, W. U. Plastic and evolutionary responses to heat stress in a temperate dung fly: negative correlation between basal and induced heat tolerance? J. Evol. Boil. 29, 900–915 (2016).
Varikou, K., Birouraki, A., Tsitsipis, I. & Sergentani, C. H. R. Effect of temperature on the fecundity of Pezothrips kellyanus (Thysanoptera: Thripidae). Ann. Entomol. Soc. Am. 105, 60–65 (2012).
Nakao, S. & Chikamori, C. Temperature-dependent wing dimorphism in a Japanese strain of tobacco thrips, Frankliniella fusca (Thysanoptera: Thripidae). Appl. Entomol. Zool. 48, 337–343 (2013).
Yashima, K. & Murai, T. Development and reproduction of a potential biological control agent, Aphelinus varipes (Hymenoptera: Aphelinidae), at different temperatures. Appl. Entomol. Zool. 48, 21–26 (2013).
Pandey, A. K. & Tripathi, C. P. M. Effect of temperature on the development, fecundity, progeny sex ratio and life-table of Campoletis chlorideae, an endolarval parasitoid of the pod borer, Helicoverpa armigera. BioControl 53, 461–471 (2008).
Moiroux, J., Brodeur, J. & Boivin, G. Sex ratio variations with temperature in an egg parasitoid: behavioural adjustment and physiological constraint. Anim. Behav. 91, 61–66 (2014).
Kang, Z. W. et al. The potential coordination of the heat-shock proteins and antioxidant enzyme genes of Aphidius gifuensis in response to thermal stress. Front. Physiol. 8, 976 (2017).
Werren, J. H. & Charnov, E. L. Facultative sex ratios and population dynamics. Nature 272, 349e350 (1978).
Shen, Y., Gong, Y. J., Gu, J., Huang, L. H. & Feng, Q. L. Physiological effect of mild thermal stress and its induction of gene expression in the common cutworm, Spodoptera litura. J. Insect Physiol. 61, 34–41 (2014).
Zhang, W., Zhao, F., Hoffmann, A. A. & Ma, C. S. A single hot event that does not affect survival but decreases reproduction in the diamondback moth, Plutella xylostella. Plos One 8, e75923 (2013).
Jørgensen, K. T., Sørensen, J. G. & Bundgaard, J. Heat tolerance and the effect of mild heat stress on reproductive characters in Drosophila buzzatii males. J. Therm. Biol. 31, 280–286 (2006).
Huang, L. H., Chen, B. & Kang, L. Impact of mild temperature hardening on thermotolerance, fecundity, and Hsp gene expression in Liriomyza huidobrensis. J. Insect Physiol. 53, 1199–1205 (2007).
Rinehart, J. P., Yocum, G. D. & Denlinger, D. L. Thermotolerance and rapid cold hardening ameliorate the negative effects of brief exposures to high or low temperatures on fecundity in the flesh fly, Sarcophaga crassipalpis. Physiol. Entomol. 25, 330–336 (2000).
Janowitz, S. A. & Fischer, K. Opposing effects of heat stress on male versus female reproductive success in Bicyclus anynana, butterflies. J. Therm. Biol. 36, 283–287 (2011).
Gillot, C. Male accessory gland secretions: Modulators of female reproductive physiology and behaviour. Annu. Rev. Entomol. 48, 163–184 (2003).
Fasolo, A. G. & Krebs, R. A. A comparison of behavioural change in Drosophila during exposure to thermal stress. Biol. J. Linn. Soc. 83, 197–205 (2004).
Zheng, H. Y. The breeding effect of Ophraella communa Lesage (Coleoptera: Chrysomelidae) in different mating mode. Jiangxi Agricultural University (2013).
Liu, X. P., He, H. M. & Xue, F. S. The effect of mating frequency and mating pattern on female reproductive fitness in cabbage beetle, Colaphellus bowringi. Entomol. Exp. Appl. 146, 379–385 (2013).
Barbosa, M., Connolly, S. R., Hisano, M., Dornelas, M. & Magurran, A. E. Fitness consequences of female multiple mating: a direct test of indirect benefits. BMC Evol. Biol. 12, 185 (2012).
Gershman, S. N. Large numbers of matings give female field crickets a direct benefit but not a genetic benefit. J. Insect Behav. 23, 59–68 (2010).
Zhou, Z. S. et al. Mating frequency positively associates with fitness in Ophraella communa. Ecol. Entomol. 40, 292–298 (2015).
Colinet, H., Sinclair, B. J., Vernon, P. & David, R. Insects in fluctuating thermal environments. Ann. Rev. Entomol. 60, 123–140 (2015).
Vos, M. J. et al. Specific protein homeostatic functions of small heat-shock proteins increase lifespan. Aging Cell 15, 217–226 (2016).
Sørensen, J. G., Kristensen, T. N., Kristensen, K. V. & Loeschcke, V. Sex specific effects of heat induced hormesis in Hsf-deficient Drosophila melanogaster. Exp. Gerontol. 42, 1123–1129 (2007).
Liang, L. N., Zhang, W., Ma, G., Hoffmann, A. A. & Ma, C. S. A single hot event stimulates adult performance but reduces egg survival in the oriental fruit moth, Grapholitha molesta. Plos One 9, e116339 (2014).
Marais, E., Terblanche, J. S. & Chown, S. L. Life stage-related differences in hardening and acclimation of thermal tolerance traits in the kelp fly, Paractora dreuxi (Diptera, Helcomyzidae). J. Insect Physiol. 55, 336–343 (2009).
Ma, G. & Ma, C. S. Effect of acclimation on heat-escape temperatures of two aphid species: implications for estimating behavioral response of insects to climate warming. J. Insect Physiol. 58, 303–309 (2012).
Silbermann, R. & Tatar, M. Reproductive costs of heat shock protein in transgenic Drosophila melanogaster. Evolution 54, 2038–2045 (2000).
David, J. R. et al. Male sterility at extreme temperatures: a significant but neglected phenomenon for understanding Drosophila climatic adaptations. J. Evolution. Biol. 18, 838–846 (2005).
Zani, P. A., Cohnstaedt, L. W., Corbin, D., Bradshaw, W. E. & Holzapfel, C. M. Reproductive value in a complex life cycle: heat tolerance of the pitcher-plant mosquito, Wyeomyia smithii. J. Evolution Biol. 18, 101–105 (2005).
Vorhees, A. S. & Bradley, T. J. Differences in critical thermal maxima and mortality across life stages of the mealworm beetle Tenebrio molitor. J. Exp. Biol. 215, 2319–2326 (2012).
Amarasekare, P. & Sifuentes, R. Elucidating the temperature response of survivorship in insects. Funct. Ecol. 26, 959–968 (2012).
Kern, P., Cramp, R. L. & Franklin, C. E. Physiological responses of ectotherms to daily temperature variation. J. Exp. Biol. 218, 3068–3076 (2015).
Tang, J. H. et al. Egg hatch and survival and development of beet webworm (Lepidoptera: Crambidae) larvae at different combinations of temperature and relative humidity. J. Econ. Entomol. 109, 1603–1611 (2016).
Chen, H. S., Solangi, G. S., Guo, J. Y., Wan, F. H. & Zhou, Z. S. Antioxidant responses of ragweed leaf beetle Ophraella communa (Coleoptera: Chrysomelidae) exposed to thermal stress. Front. Physiol. 9, 808 (2018).
Acknowledgements
We would like to thank Wanmei Yang and Tianang Lei (Hunan Agricultural University) for their help with the experiments. We also thank the editor and two anonymous reviewers for their constructive comments on the manuscript, and Springer Nature Author Services for the Language editing. This research was funded by the National Natural Science Foundation of China (No. 31672089) and the National Natural Science Foundation of China for Excellent Young Scholars (No. 31322046).
Author information
Authors and Affiliations
Contributions
Z.Z. conceived of the idea for of the project and edited the English of the manuscript. F.W. conceived of the idea for the project. X.Z. and M.L. helped perform the experiments. J.G. helped on the theoretical analysis and with revising the manuscript. G.S. helped with revising the manuscript. H.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, H., Zheng, X., Luo, M. et al. Effect of short-term high-temperature exposure on the life history parameters of Ophraella communa. Sci Rep 8, 13969 (2018). https://doi.org/10.1038/s41598-018-32262-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-32262-z
- Springer Nature Limited