Abstract
Optical thermometry based on the up-conversion intensity ratio of thermally coupled levels of rare earth ions has been widely studied to achieve an inaccessible temperature measurement in submicron scale. In this work, a novel optical temperature sensing strategy based on the energy transfer from charge transfer bands of W-O and Eu-O to Eu3+-Dy3+ ions is proposed. A series of Eu3+/Dy3+ co-doped SrWO4 is synthesized by the conventional high-temperature solid-state method. It is found that the emission spectra, emission intensity ratio of Dy3+ (572 nm) and Eu3+ (615 nm), fluorescence color, lifetime decay curves of Dy3+ (572 nm) and Eu3+ (615 nm), and relative and absolute sensitivities of Eu3+/Dy3+ co-doped SrWO4 are temperature dependent under the 266 nm excitation in the temperature range from 11 K to 529 K. The emission intensity ratio of Dy3+ (572 nm) and Eu3+ (615 nm) ions exhibits exponentially relation to the temperature due to the different energy transfer from the charge transfer bands of W-O and Eu-O to Dy3+ and Eu3+ ions. In this host, the maximum relative sensitivity Sr can be reached at 1.71% K−1, being higher than those previously reported material. It opens a new route to obtain optical thermometry with high sensitivity through using down-conversion fluorescence under ultraviolet excitation.
Similar content being viewed by others
Introduction
Recently, white light emitting diode (LED) technology has attracted much attention in the solid-state lighting industry, due to the advantages of white LEDs including power saving, long lifetime, and environmental benefit1,7,8. For examples, Das and co-authors reported the controllable white light emission from Dy3+-Eu3+ co-doped KCaBO3 phosphor6. Laguna reported the shape controlled white light emission from Dy3+-Eu3+ co-doped CaMoO4 microarchitectures7. Hirai obtained the white light emission from Dy3+-Eu3+ co-doped Sr2CeO4 8. In these works, the white light emission was controlled by changing do** concentration and host types.
It was reported that the temperature was a key parameter to adjust the emission intensity, the fluorescence intensity ratio, and emission color9,10,11,12. Berry found that the lifetime of 5D0 of Eu3+ ion was temperature dependent in Europium Tris(2,2,6,6-tetramethyl-3,5- heptanedionato)9. Morgan observed that the homogeneous linewidth of the 5D0 → 7F0 transition of Eu3+ was dependent on temperature in amorphous hosts10. Eckert observed that the phosphorescence decay lifetime of the Dy3+-transitions in Dy3+: Al2O3 showed strong temperature dependency in a temperature range from 1100 to 1500 K11. Zhou reported that the emission intensity ratio of 5D1 to 5D0 of Eu3+-doped transparent MF2 (M = Ba, Ca, Sr) glass ceramics increased with the temperature increase12. However, the temperature dependent optical property of Dy3+-Eu3+ co-doped materials has not been studied so far. It is necessary to explore the spectra and energy transfer of Dy3+-Eu3+ co-doped materials at high temperature.
From the published work on the spectra of Dy3+-Eu3+ co-doped materials, one can find that it had a little overlap of the excitation spectrum between 5D0 → 7F2 (Eu3+) and 4F9/2 → 6H13/2 (Dy3+)13, 14. It is necessary to find another ion to sensitize the Dy3+ and Eu3+ simultaneously. Notably, the charge transfer band of W-O was reported to have the wide absorption band in the ultraviolet range from 200 nm to 300 nm15,16,17. It may be a promise sensitizer to excite Dy3+ and Eu3+ simultaneously. Thus, in this work, the optical temperature property of Eu3+/Dy3+ co-doped SrWO4 are studied under 266 nm excitation. It is observed that the fluorescence intensity ratio between Eu3+ and Dy3+ emissions are strongly dependent on the temperature at the temperature range from 11 K to 529 K. The Eu3+/Dy3+ co-doped SrWO4 phosphors are proved as an excellent materials used for optical thermometry, due to its maximum value of Sr as high as 1.71% K−1.
Results
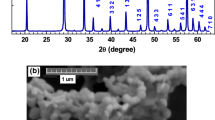
The X-ray diffraction (XRD) patterns of the SrWO4, SrWO4: 0.4 mol% Eu3+, and SrWO4: x Eu3+, 4 mol% Dy3+ (x = 0, 0.2 mol%, 0.4 mol%, 0.6 mol%, 0.8 mol%, 1 mol%) samples synthesized by high-temperature solid-state reaction method are shown in Fig. 1. The peaks of all the products can be easily indexed to tetragonal system of SrWO4, which has a I41/a space group (PDF# 08-0490, unit cell parameters: a = b = 5.416 Å, c = 11.95 Å). No trace of impurity peaks can be found when Dy3+ and Eu3+ ions are introduced into the system. Compared with the pure SrWO4, the diffraction peaks of the Eu3+, Dy3+ single-doped and Eu3+/Dy3+co-doped SrWO4 exhibit a slight shift toward high-angle side, due to substitution of Sr2+ (1.26 Å, CN = 8) ions by smaller size Dy3+ (1.03 Å, CN = 8) and Eu3+ (1.07 Å, CN = 8) ions, which revealing that Dy3+ and Eu3+ ions have been successfully doped into the system18, 19. Figure 2 shows the unit cell parameters of a (Å) and c (Å) as well as unit cell volume (Å3). It can be observed that the value of lattice parameter a (Å) decreases firstly due to substitution of Sr2+ ions by smaller size Dy3+ and Eu3+ ions, and then increases with the increase of Eu3+ concentration due to the size differences between the different valence state cations20, 21. The same tendency can be observed in the values of parameter c (Å) and volume (Å3). It reveals that Eu3+ and Dy3+ ions can be easily doped into SrWO4 lattice, and the lattice can be distorted by the do** ions.
(a) XRD patterns of the as-synthesized SrWO4, SrWO4: 0.4 mol% Eu3+, and SrWO4: x Eu3+, 4 mol% Dy3+ (x = 0, 0.2 mol%, 0.4 mol%, 0.6 mol%, 0.8 mol%, 1 mol%) phosphors. The standard data of tetragonal SrWO4 (PDF# 08-0490) is given as a reference; (b) Partially enlarged XRD patterns of the corresponding phosphors (2θ = 27–29°).
The scanning electron microscopy (SEM) image of a representative SrWO4: 0.4 mol% Eu3+, 4 mol% Dy3+ sample is shown in Fig. 3a, exhibiting sphere-like morphology with a particle size of about 1 µm. The energy dispersive spectrometer (EDS) spectrum (Fig. 3b) confirms the presence of Sr, W, O, Eu, and Dy elements, and further providing the evidence that Dy3+ and Eu3+ ions have been successfully doped into the SrWO4 host lattice.
The ultraviolet-visible diffuse reflectance spectrum of the SrWO4: 0.4 mol% Eu3+, 4 mol% Dy3+ in the range of 200–800 nm is shown in Fig. 4. A broad band and several absorption peaks corresponding to the doped ions can be observed. The broad band is located from 200 to 350 nm, corresponding to the O-W ligand-to-metal charge transfer in the WO4 2− group22, 23. Four absorption peaks located at 365, 384, 426 and 454 nm can be assigned to the intra 4 f electronic transitions of 7F0 → 5D4 (Eu3+), 7F0 → 5G2 (Eu3+), 6H15/2 → 4G11/2 (Dy3+), and 6H15/2 → 4I15/2 (Dy3+), respectively.
The PLE spectra of SrWO4: 4 mol% Dy3+, SrWO4: 0.4 mol% Eu3+, and SrWO4: 0.4 mol% Eu3+, 4 mol% Dy3+ samples are shown in Fig. 5. The PLE spectrum of SrWO4: 4 mol% Dy3+ (Fig. 5a) illustrates a broad charge transfer band centered at 247 nm from 200 nm to 280 nm and a series of sharp lines extended to visible region can be observed by monitoring at 572 nm. The broad band can be ascribed to the charge transfer from WO4 2− group to Dy3+ 24, and the seven sharp lines can be ascribed to f–f transitions of Dy3+ 4 f configuration, which are 6H15/2 → 4K13/2 (296 nm), 6H15/2 → 4K15/2 (322 nm), 6H15/2 → 4M15/2 (350 nm), 6H15/2 → 4P3/2 (365 nm), 6H15/2 → 4M21/2 (387 nm), 6H15/2 → 4G11/2 (426 nm), and 6H15/2 → 4I15/2 (450 nm), respectively25. The excitation spectrum of SrWO4: 0.4 mol% Eu3+ is shown in Fig. 5b. Monitored at 615 nm, an intense broad band can be found in the range of 250–320 nm, which is due to the Eu-O charge transfer transition24, 26. While in the range of 200–250 nm, no obvious band can be found, indicating the energy transfer from WO4 2− group to Eu3+ is negligible. Additionally, a series of sharp lines corresponding to the intra 4 f electron transitions of Eu3+ ion can also be observed, which are 360 nm (7F0 → 5D4), 380 nm (7F0 → 5L7), 393 nm (7F0 → 5L6), 414 nm (7F0 → 5D3), and 463 nm (7F0 → 5D2), respectively27. Figure 5c shows the excitation spectra of SrWO4: 0.4 mol% Eu3+, 4 mol% Dy3+ phosphors. When compared with the excitation spectrum of SrWO4: 4 mol% Dy3+ by monitoring at 572 nm, the position of broad band and the excitation peaks in both the spectra can be matched well with each other. Nevertheless, the excitation intensity of Dy3+ is greatly enhanced when Eu3+ is introduced. When monitored at 615 nm, the Eu3+ excitation intensity decreases compared with the excitation spectrum of SrWO4: 0.4 mol% Eu3+. This may be due to the energy transfer from Eu3+ to Dy3+. The apparent overlap of charge transfer band centered at about 266 nm can also be observed. Hence, 266 nm pulsed laser is selected as the excitation light source to excite Dy3+ and Eu3+ ions.
Figure 6a displays the emission curves of SrWO4: x Eu3+, 4 mol% Dy3+ (x = 0, 0.2 mol%, 0.4 mol%, 0.6 mol%, 0.8 mol%, 1 mol%) phosphors. The emission spectrum of the SrWO4: 4 mol% Dy3+ reveals a strong yellow (572 nm) emission and a blue (485 nm) emission corresponding to the 4F9/2 → 6H13/2 and 4F9/2 → 6H15/2 transition of Dy3+ ions, respectively, under the 266 nm excitation28. Two small emission peaks located at 660 and 750 nm are also observed, due to the transitions from 4F9/2 excited state to 6H11/2 and 6H9/2 ground states. And a very weak broad band in the range of 350–550 nm corresponding to the WO4 2− emission can be found. One can see that the hypersensitive electric dipole transition 4F9/2 → 6H13/2 at 572 nm dominates the spectrum, which indicates that the Dy3+ ions are placed at the sites of non-inversion symmetry5, 29. Four new emission peaks at 590, 615, 650 and 700 nm appear, due to the f-f transitions (5D0 → 7F1,2,3,4) of Eu3+ ions, along with the characteristic transitions of Dy3+ 13. The integral intensity of 572 nm and 615 nm emissions is calculated as a function of Eu3+ concentration as well as the total emissions, as shown in Fig. 6b. The emission intensity of Eu3+ (615 nm) increases with increase of the Eu3+ concentration from 0.2 mol% to 0.8 mol%, and then decreases when the concentration further increases above 0.8 mol% due to the concentration quenching effect30. The Dy3+ emission (572 nm) intensity increases with the increase of Eu3+ concentration and reaches a maximum value at Eu3+ concentration of 0.4 mol%, which can be ascribed to the energy transfer from Eu3+ to Dy3+ 31. With the continuous increasing of Eu3+ concentration, the Dy3+ emission intensity decreases, which can be attributed to the concentration quenching effect. Focusing on the total emissions intensity, when the do** concentration of Eu3+ reaches to 0.8 ml%, the strongest total emission intensity is obtained. Thus, the sample co-doped with 0.4 mol% Eu3+ and 4 mol% Dy3+ should be selected as the optimum do** concentration to study optical properties at different temperature.
(a) PL emission spectra, (b) Integral intensity of Dy3+ (572 nm), Eu3+ (615 nm) and total emission, and (c) Calculated lifetimes of 4F9/2 and 5D0 energy levels of SrWO4: x Eu3+, 4 mol% Dy3+ (x = 0, 0.2 mol%, 0.4 mol%, 0.6 mol%, 0.8 mol%, 1 mol%) phosphors under 266 nm excitation at room temperature.
The effective lifetimes of 4F9/2 and 5D0 energy levels can be expressed as32
where I(t) represents the emission intensity at time t. The decay curves of Dy3+ (4F9/2) and Eu3+ (5D0) ions at different Eu3+ concentration were recorded by monitoring at 572 nm and 615 nm, respectively. The decay curves support the existence of energy transfer progress for doped and co-doped samples. The values of lifetimes of SrWO4: x Eu3+, 4 mol% Dy3+ (x = 0, 0.2 mol%, 0.4 mol%, 0.6 mol%, 0.8 mol%, 1 mol%) phosphors were calculated by using equation (1), in Fig. 6c. The decreasing tendency of lifetimes of both 4F9/2 and 5D0 energy levels can be found with the rise of Eu3+ concentration. The Fig. 6c shows the inhomogeneous change of lifetimes of the 572 nm (Dy3+) and 615 nm (Eu3+) emissions. It means that the energy transfer from charge transfer band of W-O to Dy3+ and the energy transfer from charge transfer band of Eu-O to Eu3+ as well as energy transfer from Eu3+ to Dy3+ are different at different Eu3+ concentrations.
To further study the temperature-dependent photoluminescence performance, the emission spectra of the SrWO4: 0.4 mol% Eu3+, 4 mol% Dy3+ samples are investigated in the temperature range from 11 K to 592 K, as shown in Fig. 7a. One can see that the emission intensity of Dy3+ ions increases with the rise of temperature, while the emission intensity of Eu3+ ions decreases. The emission bands of Dy3+ ions at 572 nm (4F9/2 → 6H13/2) and Eu3+ ions at 615 nm (5D0 → 7F2) were enlarged and shown in Fig. 7b. One can find that the intensity of 572 nm (Dy3+) increases with the temperature increase, while the intensity of 615 nm (Eu3+) decreases with the temperature increase. It means that the energy transfer from charge transfer bands to Eu3+ and Dy3+ ions is temperature dependent. The Commission International de L’Eclairage (CIE) diagram (Fig. 7c) shows that the emission color of the SrWO4: 0.4 mol% Eu3+, 4 mol% Dy3+ sample can be turned from the orange-red to the yellow region with the increase of temperature from 11 K to 529 K.
In order to study the energy transfer among charge transfer bands, Eu3+ and Dy3+, the decay curves of 4F9/2 and 5D0 energy levels at different temperature were measured by monitoring at 572 nm and 615 nm, respectively, and calculated by using equation (1). The values of the effective lifetimes are shown in Fig. 7d. It can be found that the lifetimes of 4F9/2 energy level of Dy3+ ion increase with the increase of temperature, while the lifetimes of 5D0 energy level of Eu3+ ion decrease, demonstrating the different energy transfer rates from charge transfer bands to Dy3+ and Eu3+ ions33.
To study the temperature dependence of energy transfer from charge transfer bands to Eu3+-Dy3+ ions, the dynamic balance rate-equation model for the energy transfer between charge transfer bands and Eu3+-Dy3+ ions are established in Fig. 8. We supposed 7FJ(J = 0, 1, 2, 3, 4, 5, 6), 6HJ/2 (J = 15, 13, 11, 9, 7), or 1B(1T2)/1E(1T2)/1E(1T1) energy levels as a same level in the case of the fixed temperature. The energy transfer between Eu3+ and WO4 2- is neglected. The corresponding rate equations are as follows:
where σ 1 and σ 2 are the cross-section of the ground state absorption of 7FJ and 1A1, ρ 1 and ρ 2 are the incident pum** power density, N 0, N 1, N 2, N 3, N 4, N 5, N 6, N 7, and N 8 are the population densities of the levels of Eu3+, (WO4)2−, and Dy3+ respectively. β1 and β2 correspond to the energy transfer rates from 5D3 and 1B(1T2)/1E(1T2)/1E(1T1) to 4I15/2, respectively. The terms of W ij represent the nonradiative decay rates between the levels i and j, A ij is the radiative transition rates between the levels i and j.
By solving the above equations, we have
The nonradiative relaxation possibility is proportional to ref. 34
The luminescence intensity of an emission band can be expressed as
where hν i is transition energy per photon, A ij is spontaneous radiative emission probability from an i state to a j state, and N i is the state population of the i state35.
The emission intensity ratio of Dy3+ (572 nm) and Eu3+ (615 nm) ions, defined as FIR (IDy/IEu), is adopted to study the temperature-dependent photoluminescence property. Combining with above equations, the FIR (IDy/IEu) can be fitted as
where A is the fitting constant that depends on the experimental system and intrinsic spectroscopic parameter; ħω is the phonon energy; and k is a Boltzmann constant36. The absolute sensitivity and relative sensitivity can be defined as37
As displayed in Fig. 9a, the FIR data could be exponentially fitted by the equation (10) from 11 K to 529 K. The parameters A, B and ħω can be determined to be 3250.7, 0.55 and 903.8 cm−1 for the SrWO4: 0.4 mol% Eu3+, 4 mol% Dy3+ sample by using the fitting equation. The fitted phonon energy of 903.8 cm−1 is closed to the literature reported of 917.7 cm−1 38. The error of the fitted phonon energy is about 1.5%. On the basis of the equations (11) and (12), the absolute sensitivity Sa and relative sensitivity Sr are calculated and shown in Fig. 9b,c. One can see that the absolute sensitivity is as high as 0.27 K−1 at 529 K. It is much higher than the literature reported39, 40. For example, the absolute sensitivity in Eu3+ doped Gd2Ti2O7 phosphor was 0.015 K−1 41, and in Dy3+ doped GdVO4 phosphor was 0.01 K−1 42. The maximum relative sensitivity of 1.71% K−1 is obtained at 335 K. It is higher than the reported phosphors, 0.014 K−1 in Eu3+ doped CaGd2(WO4)4 scheelite43 and 0.003 °C−1 in Dy3+ doped Y4Al2O9 phosphor44. The improvement of both the relative sensitivity and absolute sensitivity of this material may be owing to different energy transfer ratio from charge transfer bands to Eu3+-Dy3+ ions at different temperatures, leading to a significant change in the emission intensity ratio.
The error analysis of measured and calculated FIR(I572/I615) is shown in Fig. 10a. One can see that the measured and the calculated FIR match well at low temperature, while the error appears at high temperature more than 400 K. The error may originated from the active nonradiative relaxation and energy transfer between Eu3+/Dy3+ ions and host39, 45. Notably, this error affects little on the values of Sa and Sr, as shown in Fig. 10b,c.
Conclusions
In this work, a series of Eu3+/Dy3+ co-doped SrWO4 phosphors were prepared by the high-temperature solid-state method. The structural property was studied by the X-Ray diffraction. The emission intensity, fluorescence color, and lifetimes of Dy3+ (572 nm) and Eu3+ (615 nm) of the SrWO4: 0.4 mol% Eu3+, 4 mol% Dy3+ are investigated in the temperature range from 11 K to 529 K under the 266 nm excitation. The emission intensity ratio of Dy3+ and Eu3+ ions was found to be temperature dependent. The maximum value of Sr can be reached 1.71% K−1 at 335 K, being higher than those previously reported material. This work opens a new route to obtain optical thermometry with high sensitivity through using down-conversion fluorescence under ultraviolet excitation.
Methods
A series of Eu3+/Dy3+ single doped and co-doped SrWO4 phosphors were prepared by the high-temperature solid-state method. According to the appropriate stoichiometric ratio, the starting materials, SrCO3 (Aldrich, 99.9%), WO3 (Aldrich, 99.9%), Eu2O3 (Aladdin, 99.99%), and Dy2O3 (Aldrich, 99.99%) were weighted and ground thoroughly in an agate mortar for 30 minutes with ethanol. Then the homogenous mixture was collected into a crucible and sintered at 1000 °C for 4 hours. After cooling to the room temperature, the obtained white samples were ground to powder for further investigation.
The obtained products were characterized by X-ray diffraction (XRD) using a Philips X’Pert MPD (Philips, Netherlands) X-ray diffractometer at 40 kV and 30 mA. All patterns are recorded in the range of 10–90° with a step size of Δ2θ = 0.02. The morphology, particle size and energy dispersive spectrometer (EDS) of the phosphor are characterized by scanning electron microscope (SEM) system (JSM-6490, JEOL Company). The ultraviolet-visible diffuse reflectance spectrum is recorded using a V-670 (JASCO) UV-vis spectrophotometer. The photoluminescence excitation (PLE) spectra are recorded by a Pjoton Technology International (PTI, USA) fluorimeter with a 60 W Xe-arc lamp as the excitation light source at room temperature. The photoluminescence (PL) spectra and decay lifetimes are collected by a 266 nm-pulsed laser with a pulse width of 5 ns and a repetition rate of 10 Hz (Spectron Laser Sys. SL802G).
References
Kim, J. S. et al. Warm-white-light emitting diode utilizing a single-phase full-color Ba3MgSi2O8: Eu2+, Mn2+ phosphor. Applied Physics Letters 84, 2931–2933 (2004).
Ye, S., **ao, F., Pan, Y. X., Ma, Y. Y. & Zhang, Q. Y. Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties. Materials Science and Engineering: R: Reports 71, 1–34 (2010).
**e, R. J., Hirosaki, N., Kimura, N., Sakuma, K. & Mitomo, M. 2-phosphor-converted white light-emitting diodes using oxynitride/nitride phosphors. Applied physics letters 90, 191101 (2007).
Kuznetsov, A. S., Nikitin, A., Tikhomirov, V. K., Shestakov, M. V. & Moshchalkov, V. V. Ultraviolet-driven white light generation from oxyfluoride glass co-doped with Tm3+-Tb3+-Eu3+. Applied Physics Letters 102, 161916 (2013).
Wang, X., Zheng, J., Xuan, Y. & Yan, X. Optical temperature sensing of NaYbF4: Tm3+@SiO2 core-shell micro-particles induced by infrared excitation. Optics express 21, 21596–21606 (2013).
Das, S., Reddy, A. A., Babu, S. S. & Prakash, G. V. Controllable white light emission from Dy3+–Eu3+ co-doped KCaBO3 phosphor. Journal of materials science 46, 7770 (2011).
Laguna, M., Nuñez, N. O., Becerro, A. I., & Ocaña, M. Morphology control of uniform CaMoO4 microarchitectures and development of white light emitting phosphors by Ln do** (Ln = Dy3+, Eu3+). CrystEngComm (2017).
Hirai, T. & Kawamura, Y. Preparation of Sr2CeO4: Eu3+, Dy3+ white luminescence phosphor particles and thin films by using an emulsion liquid membrane system. The Journal of Physical Chemistry B 109, 5569–5573 (2005).
Berry, M. T., May, P. S. & Xu, H. Temperature dependence of the Eu3+ 5D0 lifetime in europium tris (2, 2, 6, 6-tetramethyl-3, 5-heptanedionato). The Journal of Physical Chemistry 100, 9216–9222 (1996).
Morgan, J. R. & El-Sayed, M. A. Temperature dependence of the homogeneous linewidth of the 5D0-7F0 transition of Eu3+ in amorphous hosts at high temperatures. Chemical Physics Letters 84, 213–216 (1981).
Eckert, C., Pflitsch, C. & Atakan, B. Dy3+: Al2O3 and (Dy3+ + Cr3+): Al2O3 films for temperature sensor applications derived by thermal CVD and sol-gel techniques. ECS Transactions 25, 1293–1300 (2009).
Zhou, B., Bu, Y. Y., Meng, L., Yan, X. H. & Wang, X. F. Temperature‐controlled down‐conversion luminescence behavior of Eu3+‐doped transparent MF2 (M = Ba, Ca, Sr) glass ceramics. Luminescence. (2016).
Dutta, S. & Sharma, S. K. Energy transfer between Dy3+ and Eu3+ in Dy3+/Eu3+-codoped Gd2MoO6. Journal of Materials Science 51, 6750–6760 (2016).
Liu, Y., Liu, G., Dong, X., Wang, J. & Yu, W. Tunable photoluminescence and magnetic properties of Dy3+ and Eu3+ doped GdVO4 multifunctional phosphors. Physical Chemistry Chemical Physics 17, 26638–26644 (2015).
Bharat, L. K. & Yu, J. S. Synthesis and luminescent properties of Eu3+ activated SrWO4 nanocrystalline microspheres. Journal of nanoscience and nanotechnology 13, 8239–8244 (2013).
Barros, B. S., De Lima, A. C., Da Silva, Z. R., Melo, D. M. A. & Alves-Jr., S. Synthesis and photoluminescent behavior of Eu3+-doped alkaline-earth tungstates. Journal of Physics and Chemistry of Solids 73, 635–640 (2012).
Neeraj, S., Kijima, N. & Cheetham, A. K. Novel red phosphors for solid-state lighting: the system NaM(WO4)2−x(MoO4)x: Eu3+(M = Gd, Y, Bi). Chemical Physics Letters 387, 2–6 (2004).
Mota, N. et al. Hydrogen production by autothermal reforming of methane over lanthanum chromites modified with Ru and Sr. International Journal of Hydrogen Energy 41, 19373–19381 (2016).
Sharma, K. G. & Singh, N. R. Synthesis and luminescence properties of CaMO4: Dy3+(M = W, Mo) nanoparticles prepared via an ethylene glycol route. New Journal of Chemistry 37, 2784–2791 (2013).
Singh, B. P., Singh, J. & Singh, R. A. Luminescence properties of Eu3+-activated SrWO4 nanophosphors-concentration and annealing effect. RSC Advances 4, 32605–32621 (2014).
Tozri, A., Bejar, M., Dhahri, E. & Hlil, E. K. Structural and magnetic characterisation of the perovskite oxides La0.7Ca0.3−xNaxMnO3. Central European Journal of Physics 7, 89–95 (2009).
Ahmad, G. et al. Rapid, Room‐Temperature Formation of Crystalline Calcium Molybdate Phosphor Microparticles via Peptide‐Induced Precipitation. Advanced Materials 18, 1759–1763 (2006).
Li, L. Z., Yan, B., Lin, L. X. & Zhao, Y. Solid state synthesis, microstructure and photoluminescence of Eu3+ and Tb3+ activated strontium tungstate. Journal of Materials Science: Materials in Electronics 22, 1040–1045 (2011).
Liu, Y., Liu, G., Wang, J., Dong, X. & Yu, W. Single-component and warm-white-emitting phosphor NaGd(WO4)2: Tm3+, Dy3+, Eu3+: synthesis, luminescence, energy transfer, and tunable color. Inorganic chemistry 53, 11457–11466 (2014).
Watras, A., Dereń, P. J. & Pązik, R. Luminescence properties and determination of optimal RE3+(Sm3+, Tb3+ and Dy3+) do** levels in the KYP2O7 host lattice obtained by combustion synthesis. New Journal of Chemistry 38, 5058–5068 (2014).
Ju, Z., Wei, R., Gao, X., Liu, W. & Pang, C. Red phosphor SrWO4: Eu3+ for potential application in white LED. Optical Materials 33, (909–913 (2011).
Lin, J., Su, Q., Wang, S. & Zhang, H. Influence of crystal structure on the luminescence properties of bismuth (III), europium (III) and dysprosium (III) in Y2SiO5. Journal of Materials Chemistry 6, 265–269 (1996).
Luo, L., Huang, F. Y., Dong, G. S., Wang, Y. H. & Hu, Z. F. & Chen,J. White Light Emission and Luminescence Dynamics in Eu3+/Dy3+ Codoped ZnO Nanocrystals. Journal of Nanoscience and Nanotechnology 16, 619–625 (2016).
Sudarsan, V., Van Veggel, F. C., Herring, R. A. & Raudsepp, M. Surface Eu3+ ions are different than “bulk” Eu3+ ions in crystalline doped LaF3 nanoparticles. Journal of Materials Chemistry 15, 1332–1342 (2005).
Yang, H. M., Shi, J. X., Liang, H. B. & Gong, M. L. A novel red phosphor Mg2GeO4 doped with Eu3+ for PDP applications. Materials Science and Engineering: B 127, 276–279 (2006).
Wan, J. et al. Energy transfer and colorimetric properties of Eu3+/Dy3+ co-doped Gd2(MoO4)3 phosphors. Journal of Alloys and Compounds 496, 331–334 (2010).
Chen, D., Wang, Y., Yu, Y., Huang, P. & Weng, F. Near-infrared quantum cutting in transparent nanostructured glass ceramics. Optics letters 33, 1884–1886 (2008).
Nikolić, M. G., Antić, Ž., Ćulubrk, S., Nedeljković, J. M. & Dramićanin, M. D. Temperature sensing with Eu3+ doped TiO2 nanoparticles. Sensors and Actuators B: Chemical 201, 46–50 (2014).
Wang, X. et al. Excitation powder dependent optical temperature behavior of Er3+ doped transparent Sr0.69La0.31F2.31 glass ceramics. Optics Express 24, 17792–17804 (2016).
**ao, S., Yang, X., Liu, Z. & Yan, X. H. Up-conversion in Er3+: Y2O3 Nanocrystals Pumped at 808 nm. Journal of applied physics 96, 1360–1364 (2004).
Shinn, M. D., Sibley, W. A., Drexhage, M. G. & Brown, R. N. Optical transitions of Er3+ ions in fluorozirconate glass. Physical Review B 27, 6635 (1983).
Dramićanin, M. D. Sensing temperature via downshifting emissions of lanthanide-doped metal oxides and salts. A review. Methods and Applications in Fluorescence 4, 042001 (2016).
Xu, B. et al. Controlled synthesis and novel luminescence properties of string SrWO4: Eu3+ nanobeans. Dalton Transactions 43, (11493–11501 (2014).
Wang, X. et al. Optical temperature sensing of rare-earth ion doped phosphors. Rsc Advances 5, 86219–86236 (2015).
Cao, Z. et al. Temperature dependent luminescence of Dy3+ doped BaYF5 nanoparticles for optical thermometry. Current Applied Physics 14, 1067-1071 (2014).
Lojpur, V., Ćulubrk, S. & Dramićanin, M. D. Ratiometric luminescence thermometry with different combinations of emissions from Eu3+ doped Gd2Ti2O7 nanoparticles. Journal of Luminescence 169, 534–538 (2016).
Nikolić, M. G., Jovanović, D. J. & Dramićanin, M. D. Temperature dependence of emission and lifetime in Eu3+-and Dy3+-doped GdVO4. Applied optics 52, 1716–1724 (2013).
Meert, K. W. et al. Energy transfer in Eu3+ doped scheelites: use as thermographic phosphor. Optics express 22, A961–A972 (2014).
Boruc, Z., Kaczkan, M., Fetlinski, B., Turczynski, S. & Malinowski, M. Blue emissions in Dy3+ doped Y4Al2O9 crystals for temperature sensing. Optics letters 37, 5214–5216 (2012).
Wade, S. A., Collins, S. F. & Baxter, G. W. Fluorescence intensity ratio technique for optical fiber point temperature sensing. Journal of Applied physics 94, 4743–4756 (2003).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03029432) National Natural Science Foundation of China (NSFC) (11404171),and the Six Categories of Summit Talents of Jiangsu Province of China (2014-XCL-021).
Author information
Authors and Affiliations
Contributions
X.W. and H.S. developed the idea and supervised the project. J.W. did all the synthetic experiments and performed measurements. Y.B. analyzed the structure and spectra properties. All authors discussed the results and contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Bu, Y., Wang, X. et al. A novel optical thermometry based on the energy transfer from charge transfer band to Eu3+-Dy3+ ions. Sci Rep 7, 6023 (2017). https://doi.org/10.1038/s41598-017-06421-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06421-7
- Springer Nature Limited
This article is cited by
-
Photoluminescence properties of YNbO4:RE3+ (RE = Dy or Eu) phosphors for anti-counterfeiting applications
Applied Physics A (2022)
-
Sensors for optical thermometry based on luminescence from layered YVO4: Ln3+ (Ln = Nd, Sm, Eu, Dy, Ho, Er, Tm, Yb) thin films made by atomic layer deposition
Scientific Reports (2019)